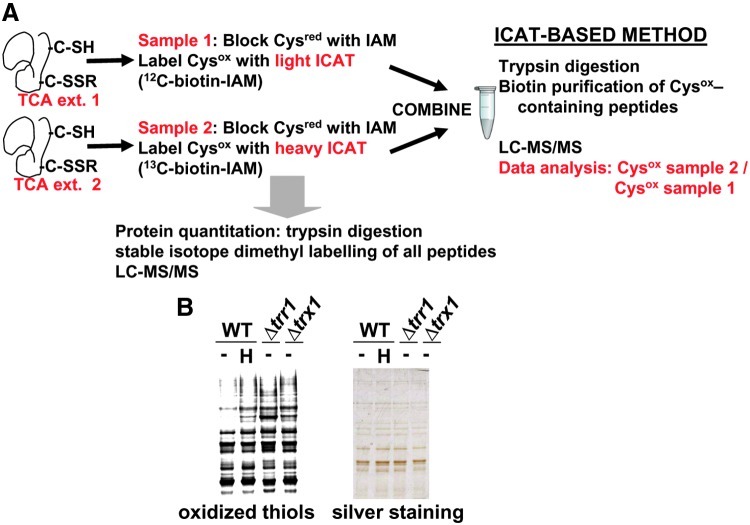
FIG. 1.
Isotope-coded affinity tag (ICAT) strategy for studying the in vivo status of reversibly oxidized cysteines in different strain backgrounds and conditions. (A) Schematic representation of the ICAT methodology. Trichloroacetic acid (TCA) protein extracts were obtained for each pair of samples to be analyzed at a time. Thiols (Cysred) in the extracts were alkylated with iodoacetamide (IAM). Upon reduction of oxidized thiols (Cysox), resulting thiols were alkylated with either light (12C-biotin-IAM) or heavy (13C-biotin-IAM) ICAT reagent. Labeled protein extracts were then mixed and digested with trypsin. ICAT-labeled peptides were affinity purified through streptavidin columns, fractionated by liquid chromatography, and analyzed by mass spectrometry (LC-MS/MS). To quantify individual proteins by dimethyl labeling, small fractions of protein extracts were digested with trypsin, and resulting peptides were labeled at their amino groups with light or heavy formaldehyde (dimethyl labeling). Resulting peptides were mixed and fractionated by LC-MS/MS. (B) Labeling of reversibly oxidized cysteines for 1D electrophoresis. Free thiols in TCA protein extracts of untreated (−) or treated (0.2 mM H2O2 for 30 s; H) cultures of strains 972 (WT), SG167 (Δtrr1), and MJ15 (Δtrx1) were alkylated with iodoacetamide. Upon reduction of oxidized thiols, resulting thiols were alkylated with a fluorescently labeled iodoacetamide derivative. Samples were analyzed by fluorescent 1D gel electrophoresis (oxidized thiols) and with silver staining, as a control of protein loading. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)