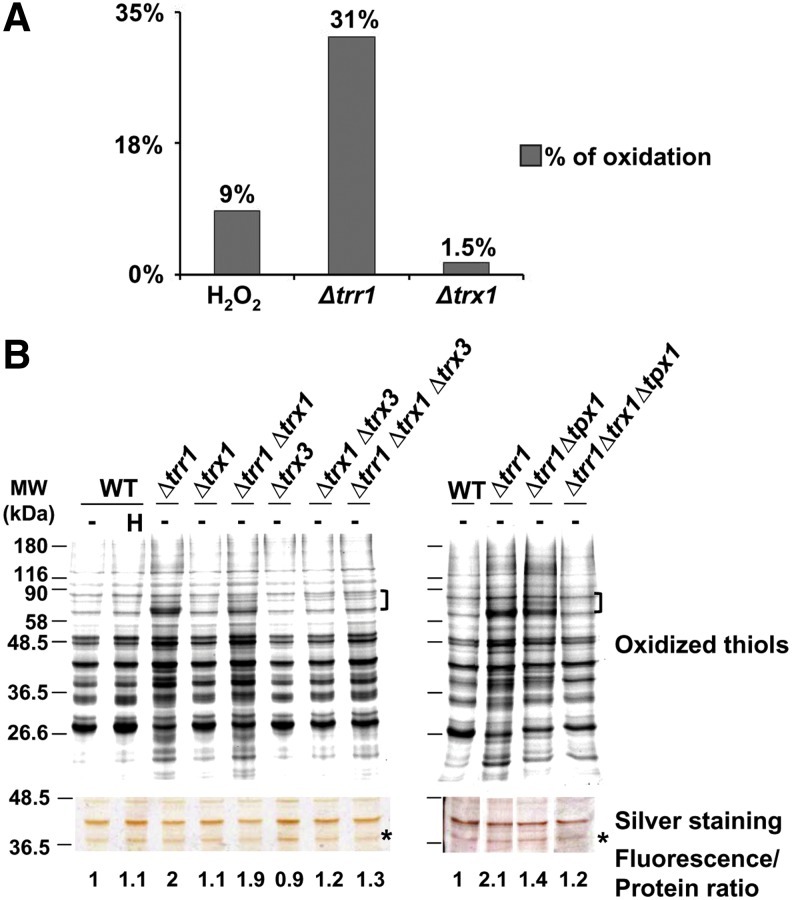
FIG. 3.
General oxidation of thiols in Δtrr1 cells is dependent on the presence of oxidized cytoplasmic Trx1 and Trx3 and/or Tpx1. (A) Percentage of oxidized cysteines in the different strains according to ICAT data. For each peptide in each biological condition (wild-type treated with H2O2; untreated Δtrr1; or untreated Δtrx1) a ratio of oxidation was always obtained comparing to untreated wild-type cells (WT unt.). An oxidation average ratio was calculated for those peptides having values>1.5-fold in 2 out of 3 biological replicates (for wild-type treated with H2O2 and untreated Δtrr1 samples), or>1.5-fold in 2 out of 2 biological replicates (for Δtrx1 samples). For those peptides having values of protein quantification by dimethyl labeling, a ratio was calculated as oxidation average ratio/protein levels, and only those having this ratio>1.5-fold are included in this graph. For peptides not displaying values on protein concentration, we eliminated those regulated by Pap1, and those having an oxidation average ratio>1.5-fold are included in this graph. Bars represent the percentage of cysteine-containing peptides, which fulfill the previous criteria for each experimental condition. (B) Role of the Trx/Trr system in the homeostasis of cysteine protein oxidation. Free thiols from TCA protein extracts from untreated (−) or treated (0.2 mM H2O2, 30 s; H) strains 972 (WT), SG167 (Δtrr1), MJ15 (Δtrx1), PG22 (Δtrr1 Δtrx1), SG189 (Δtrx3), IC76 (Δtrx1 Δtrx3), SG185 (Δtrr1 Δtrx1 Δtrx3), SG164 (Δtrr1 Δtpx1), and SG170 (Δtrr1 Δtrx1 Δtpx1) were processed as described in Figure 1B. The intensity of selected fluorescent labeled proteins, indicated in the figure with left braces, was quantified with ImageQuant. The intensity of selected proteins of the silver staining gel, indicated with an asterisk, was quantified with ImageJ. Fluorescence to protein ratios were calculated and are indicated in the figure. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)