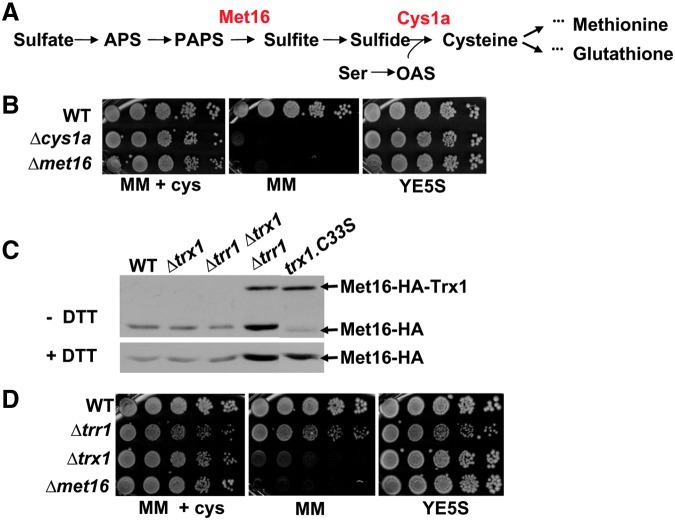
FIG. 4.
In the absence of its reductase, Trx1 is able to recycle substrates at the expense of an alternative electron donor. (A) Cysteine biosynthesis pathway in Schizosaccharomyces pombe. The enzymatic roles of Met16 and Cys1a are indicated. (B) Exponentially growing 972 (WT), SG178 (Δcys1a), and SG171 (Δmet16) strains were serially diluted and spotted on minimal medium (MM), MM containing 0.66 mM cysteine (MM+cys), and YE5S plates. (C) Mixed disulfide of Met16 with Trx1. Immunodetection of Met16-HA from TCA extracts of strains SG54 (WT), SG71 (Δtrx1), SG78 (Δtrr1 Δtrx1), SG59 (Δtrr1), and SG181 (trx1.C33S), under nonreducing (−DTT, upper panel) and reducing (+DTT, lower panel) electrophoresis. Since cysteine 33 in Trx1 resolves the mixed disulfides with its substrates, the Trx1.C33S mutant allows in vivo trapping of an intermolecular disulfide of Trx1 with Met16. Arrows indicate Met16-HA and Met16-HA covalently linked to Trx1. (D) S. pombe cells lacking Trx1 are auxotrophic for cysteine, whereas S. pombe cells lacking Trr1 are not. Exponentially growing cultures of strains 972 (WT), SG167 (Δtrr1), MJ16 (Δtrx1), and SG171 (Δmet16) were serially diluted and spotted on MM, MM containing 0.66 mM cysteine (MM+cys), and YE5S plates. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)