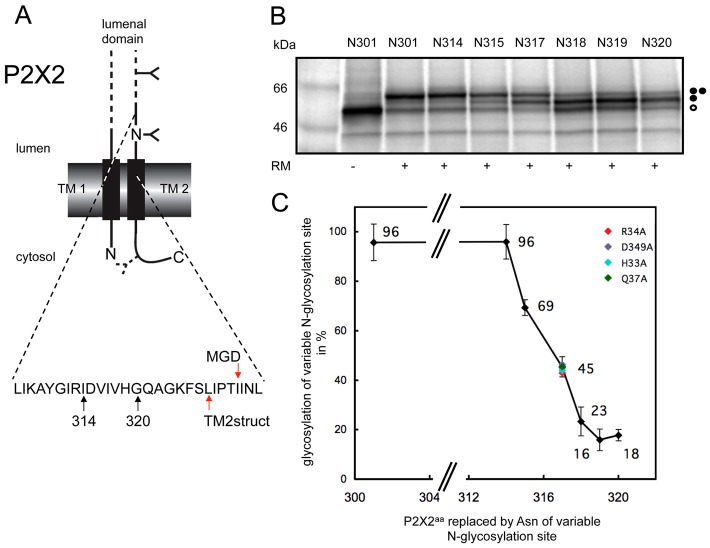
Fig. 1.
Defining the extra-cytoplasmic boundary of P2X2-TM2 using minimal glycosylation distance (MGD) mapping. (A) Wild-type rat P2X2 was engineered to contain one readily accessible N-glycosylation site at residue 182, together with a second potential site introduced at a range of locations between residues 301 and 320 (see branched structures), to enable the location of the extra-cytoplasmic boundary of the second TM of P2X2 to be estimated. A third cryptic N-glycosylation site that was introduced as a reporter at residue 381 in the cytoplasmic tail of P2X2 (see dashed line branched structure), although no evidence for its modification was observed. Arrows labelled 314 and 320 indicate the luminal region of P2X2 that was analysed by N-glycosylation scanning (see also B), while the two red arrows indicate the extra-cytoplasmic boundary of P2X2 TM2 as deduced from the high-resolution crystal structure (labelled TM2struct) and the empirical minimal glycosylation distance approach (MGD). (B) The levels of singly glycosylated (filled circle) and doubly glycosylated (two filled circles) forms of each P2X2 variant were quantified by calculating the peak areas of the corresponding radiolabelled proteins from a 2D intensity profile of each lane. The amount of the doubly glycosylated species was then expressed as a percentage of the total glycosylated protein present (i.e. singly and doubly glycosylated forms). Unglycosylated proteins are indicated by an open circle. (C) The values obtained from quantification of the products shown in B were plotted as a function of the relative locations of the variable N-glycosylation site, and the point at which 50% modification occurred was used to provide an estimate of a location 14 residues form the extra-cytoplasmic boundary TM2 (cf. Nilsson and von Heijne, 1993; Kauko et al., 2010). The outcome of altering key residues in TM1 and TM2 to alanine was investigated by analysing the N-glycosylation efficiency of residue N317 (see colour coding). For wild-type TMs (black diamonds), the values shown are the mean of three independent experiments with the standard deviation indicated.