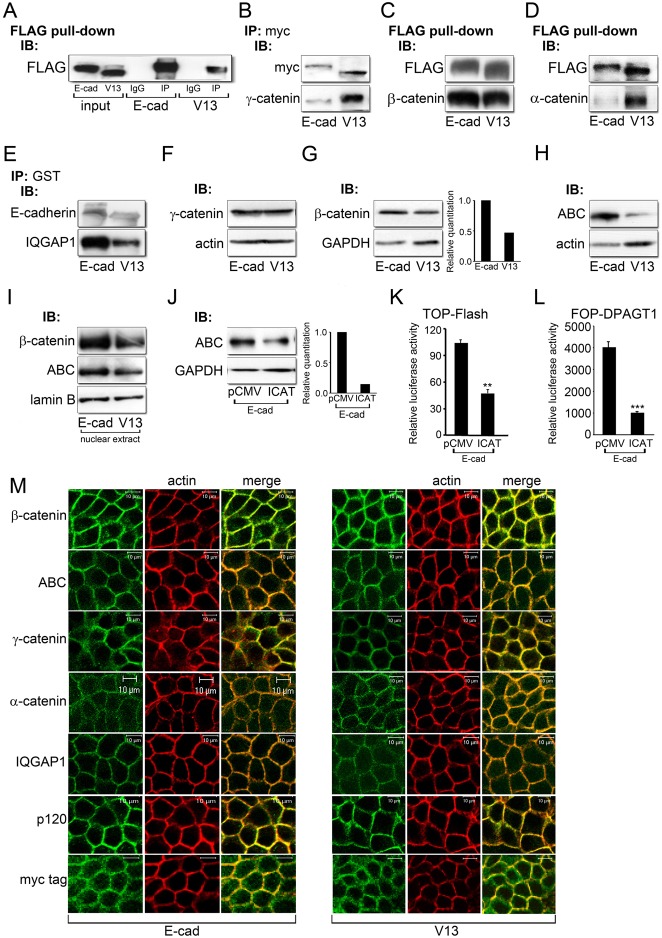
Fig. 5.
The hypoglycosylated E-cadherin, V13, inhibits canonical Wnt signaling through different mechanisms. (A) Representative control for immunoprecipitation experiments. E-cadherins were immunoprecipitated with an antibody to FLAG from E-cad and V13 cells and their association with either control IgG isotypes or FLAG was analyzed by immunoblot. To assure specificity, all immunoprecipitation studies routinely included IgG controls. (B) E-cadherins were immunoprecipitated with an antibody to Myc tag from E-cad and V13 cells and their association with γ-catenin was assessed by immunoblot. (C) FLAG pull-down samples from E-cad and V13 cells and their association with β-catenin was examined by immunoblot. (D) FLAG pull-down samples from E-cad and V13 cells and their association with α-catenin was analyzed by immunoblot. (E) E-cadherins were immunoprecipitated with an antibody to GST from E-cad and V13 cells and their association with IQGAP1 was assessed by immunoblot. (F) Immunoblot of γ-catenin expression in E-cad and V13 cells. (G) Immunoblot of β-catenin expression in E-cad and V13 cells. Bar graph: fold change in β-catenin levels after normalization to GAPDH. (H) Immunoblot of ABC protein levels in E-cad and V13 cells. (I) Immunoblot of ABC and β-catenin levels in nuclear extracts from E-cad and V13 cells. (J) Immunoblot of ABC in E-cad cells transfected with either the pCMV control vector or an active β-catenin inhibitor, ICAT. Bar graph: fold change in ABC levels after normalization to GAPDH. (K) Luciferase reporter activity from the TOP-Flash vector in E-cad cells transfected with either the pCMV control vector or ICAT (**P<0.005). (L) Luciferase reporter activity from the FOP-DPAGT1 vector in E-cad cells transfected with either the pCMV control vector or ICAT (***P<0.001). (M) Immunofluorescence localization of β-catenin, ABC, γ-catenin, α-catenin, IQGAP1, p120 and Myc tag in E-cad and V13 cells counterstained for F-actin. Scale bars, 10 µm.