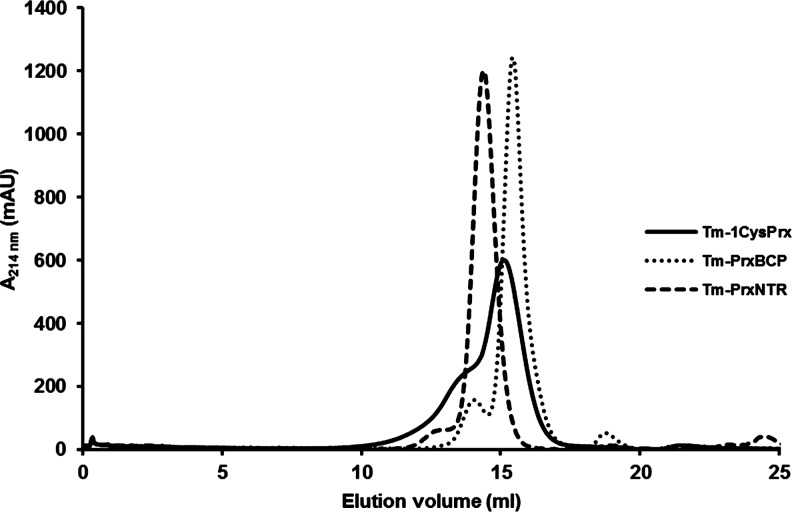
FIG. 4.
Oligomerization state of Thermotoga maritima Prxs. Analytical gel filtration of purified recombinant proteins was performed on a Superdex S200 column, pre-equilibrated with 30 mM Tris HCl pH 8.0, 200 mM NaCl. 250 μl samples of reduced proteins, at a concentration of 0.4 μg/μl, were loaded, and elution was carried out at a flow rate of 0.5 ml/min. TmPrx6 (theoretical mass of ca. 24 kDa) separated into two peaks: a major one with an estimated molecular mass of 41 kDa and a minor one of 80 kDa, suggesting that enzyme preparation contains both dimeric and tetramer forms. TmPrxBCP eluted as a major peak corresponding to a molecular mass of 35 kDa, which is consistent with a dimer, a monomer having a predicted molecular mass of 17.3 kDa. A minor peak at 70 kDa likely corresponding to a small fraction of tetramer is also visible. For as-isolated TmPrxNtr (theoretical monomeric mass of 37.2 kDa), an apparent molecular weight of 386 kDa, which is best interpreted as indicating a decamer, was found using DLS performed under non-reducing conditions. Pre-reducing TmPrxNtr decreased the apparent molecular mass to a value between 62 kDa, estimated by gel filtration, and 70 kDa, estimated by DLS, which is consistent with a dimeric form. In contrast, TmPrxNtrC40S displayed an apparent molecular mass of 74 kDa both in the presence and absence of reductants. DLS, dynamic light scattering.