Summary
A modified Delphi process assessed current multiple sclerosis (MS) practice patterns for secondary and primary progressive MS (secondary progressive MS [SPMS] and primary progressive MS [PPMS]). In early 2011, 2 sequential, case-based surveys were administered to 75 US MS specialists to assess treatment practices and patient management. Respondents were from geographically diverse US academic (42%) and community (58%) treatment centers. There was consensus (≥75% agreement in responses) to switch disease-modifying therapies for a patient with SPMS with both MRI activity and disability progression (95%), but no consensus on treatment selection. For PPMS, responses supported diagnosis using spinal MRI (100%) and lumbar puncture (75%) and treatment initiation in patients with brain gadolinium-enhancing lesions with or without spinal cord lesions (85%); however, there was no consensus on treatment initiation with spinal cord lesions alone or initial therapy. The lack of agreement among US MS experts on the best treatment approaches for SPMS or PPMS highlights the need for effective therapies.

Multiple sclerosis (MS) is one of the most common neurologic disorders in young adults, but its clinical course is unpredictable and highly variable among individual patients.1 Approximately 80%–90% of patients with MS initially develop relapsing-remitting MS (RRMS).2 Over approximately 10 years, an estimated 50% of untreated patients with RRMS will experience progressive neuronal damage, worsening of symptoms, a decline in both physical and cognitive abilities, and recategorization of their disease as secondary progressive MS (SPMS).3 Approximately 15% of patients with MS experience primary progressive MS (PPMS),2 which is characterized by few or no brain MS lesions and evidence of spinal atrophy in the absence of relapses.4 Progressive MS disease courses are associated with a worse prognosis and decreased patient survival compared with other forms of MS.5 There is currently no class I evidence for initiating treatment for patients with SPMS or PPMS.5,6 Indeed, limited evidence exists in any form for effective treatment of SPMS or PPMS.
Until recently, only interferon-β (IFNβ) preparations, glatiramer acetate (GA), and mitoxantrone were available as US Food and Drug Administration (FDA)–approved disease-modifying therapies (DMTs) for MS. Although one study reported IFNβ reducing SPMS relapse incidence and slowing disease progression,7 most trials and meta-analyses of reported trial data have concluded that IFNβ therapies have either minimal or no efficacy in preventing disability progression in SPMS or PPMS.8,9 Additional DMTs introduced in recent years10–12 and MS therapeutics in development13 may hold promise for patients with progressive MS, but clinical trial data are lacking. In the absence of sufficient clinical trial data, widely held opinion drawn from a survey of experts in the field can help guide patient care. A modified Delphi process was used to obtain MS expert consensus opinions on the diagnosis, treatment, and management of MS based on individual clinical experience with FDA-approved MS therapies at the time the surveys were administered. This report describes current US approaches to the treatment of SPMS and PPMS.
METHODS
Survey participants were MS experts identified from the Consortium of Multiple Sclerosis Centers (CMSC) list of 207 MS treatment centers in the United States. Participants were self-selected based on willingness to complete the surveys. All survey responses were deidentified prior to data analyses, and no individual responses were known to the Steering Committee (O.K., A.E.M., C.T., and J.T.P.).
A modified Delphi process was used to assess the current practice patterns of study respondents in the diagnosis and treatment of MS in the United States. The 2 serial, case-based surveys contained questions designed by the Steering Committee to determine the influence and use of various diagnostic and clinical parameters in clinical decisions pertaining to DMT initiation, switching, and choice (table 1). Both surveys were Web-based, with access through a secure e-mail link. Survey results for SPMS and PPMS are presented below, while data for CIS, RRMS, and RIS are presented in a separate article on pages 48–57 of this issue. These surveys were developed independent of any input from the CMSC, and they were distributed 4 months after FDA approval of fingolimod for US marketing and prior to publication of the 2010 revised MS diagnostic criteria.14
Table 1.
Topics addressed in surveys using a combination of dichotomous (yes–no agreement), ranking, and open response questions
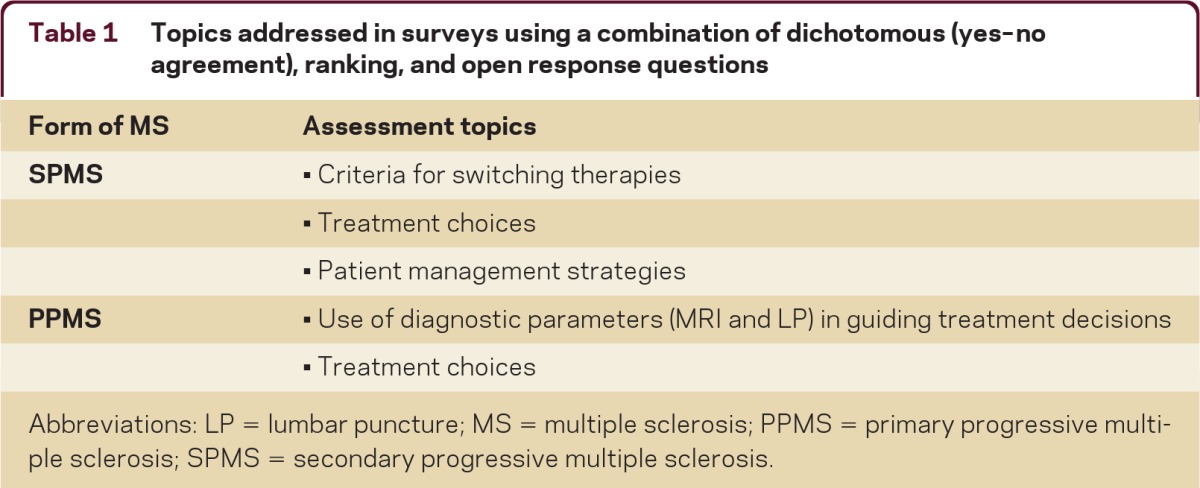
Abbreviations: LP = lumbar puncture; MS = multiple sclerosis; PPMS = primary progressive multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
For this study, consensus among respondents was defined a priori by the Steering Committee as at least 75% agreement. The Steering Committee also differentiated among consensus (≥75%), unanimous (100%), and majority (≥50%) opinion. In the current study, 83% of survey respondents also considered 75% agreement a realistic and practical threshold for consensus. Results were compiled separately for all respondents to each survey, and summary statistics were generated using Microsoft Excel. No formal statistical analyses were performed.
Detailed methods are provided in appendix e-1 at neurology.org/cp.
RESULTS
Respondents came from both academic (42%) and community (58%) MS treatment centers and represented a diverse range of US geographic regions and communities (figure e-1). Seventy-five individuals completed the first survey, and 71 of those also completed the second survey.
Practice patterns for SPMS
Case: A 48-year-old woman with MS on a DMT for 14 years has progressively lost mobility independent of clinical relapses (cane-dependent for last 3 years; last attack 4 years ago). A repeat MRI shows 5 new brain T2 lesions compared with an MRI 12 months earlier, and she reports slowly worsening cognitive and ambulatory problems.
There was consensus (95%) among respondents to switch therapy for a patient with SPMS with both MRI activity and gradual progression. When asked which DMT was the respondent's preferred treatment option when switching from an IFNβ or GA, and assuming that the patient had been treated with only one therapy, the majority (55%–63%) of respondents selected natalizumab as an alternate therapy (table 2). “Other” therapy choices (open response) included switching to a chemotherapy drug such as mitoxantrone, cyclophosphamide, or the anti-CD20 antibody rituximab, or the use of an immunosuppressant drug such as mycophenolate or methylprednisolone.
Table 2.
Therapy choices for patients with SPMS and both clinical and MRI evidence for progression
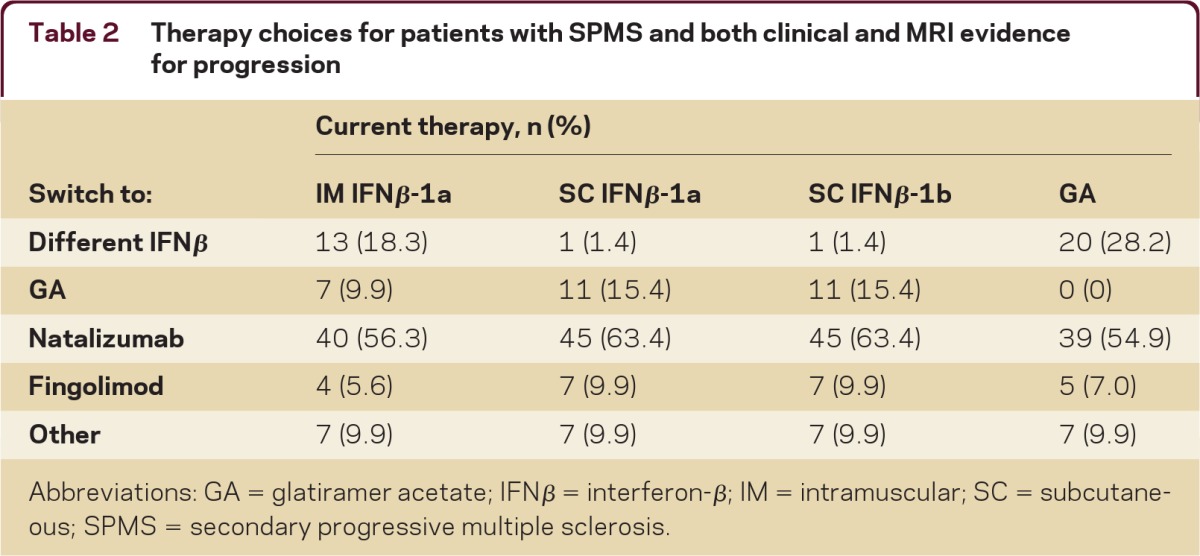
Abbreviations: GA = glatiramer acetate; IFNβ = interferon-β; IM = intramuscular; SC = subcutaneous; SPMS = secondary progressive multiple sclerosis.
The majority of respondents (60%) stated that clinical evidence of disability progression, such as changes in cognition, ambulation, gait, vision, bladder function, bowel function, and ability to complete activities of daily living, was sufficient evidence of treatment failure to prompt a DMT switch. There was consensus (87%) among respondents that breakthrough disease in the form of relapses and MRI activity should not be treated differently in SPMS vs RRMS. In open responses, comprehensive patient care was emphasized for SPMS, with symptomatic management through targeted physical therapy, dalfampridine for gait difficulties, and baclofen or tizanidine for spasticity.
Practice patterns for PPMS
Case: A 49-year-old man presents with progressive gait problems over the prior 18 months. A brain MRI reveals lesions consistent with MS, and he is diagnosed with PPMS. The patient is treatment naive.
Respondents unanimously favored performing a spinal MRI to confirm the patient's diagnosis, and 75% would perform a lumbar puncture (LP) as well. If the spinal cord MRI results and the CSF were consistent with MS, the majority (56%) of respondents would initiate treatment with a DMT. Initial treatment preferences (table 3, left column) were highly variable and included other therapies such as mitoxantrone, rituximab, cyclophosphamide, mycophenolate, and methylprednisolone.
Table 3.
Therapy choices for patients with PPMS and brain MRI Gd+ lesions
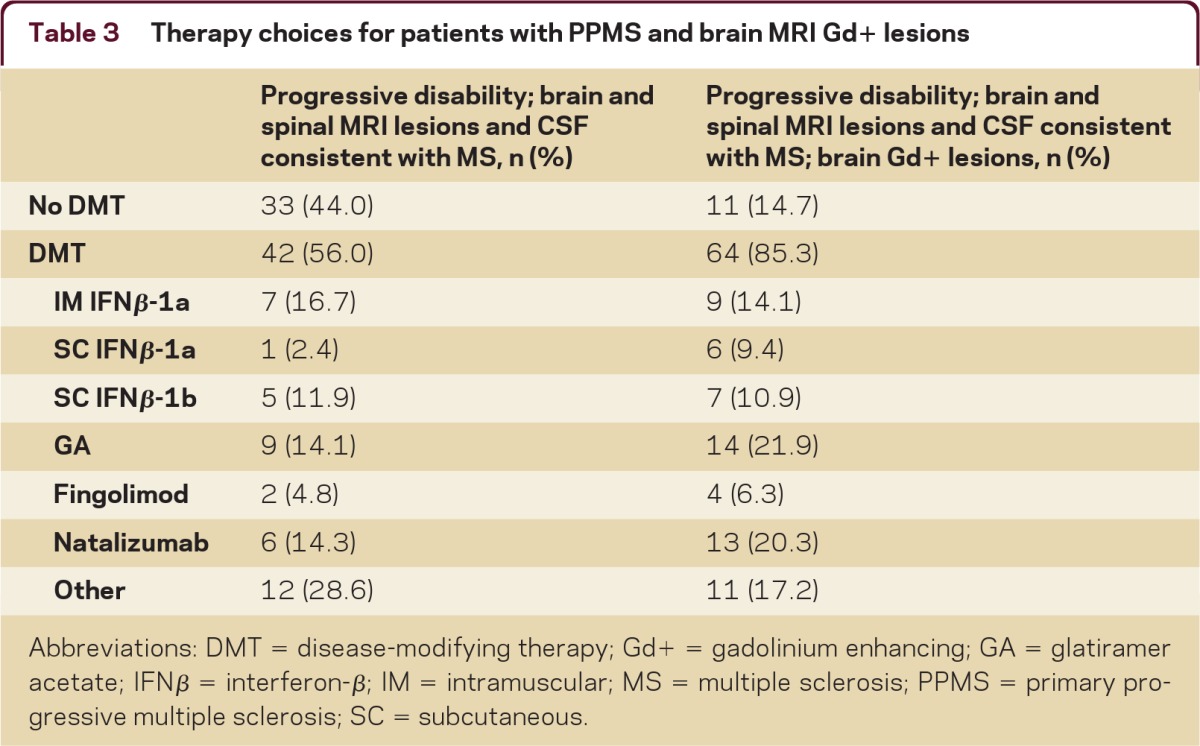
Abbreviations: DMT = disease-modifying therapy; Gd+ = gadolinium enhancing; GA = glatiramer acetate; IFNβ = interferon-β; IM = intramuscular; MS = multiple sclerosis; PPMS = primary progressive multiple sclerosis; SC = subcutaneous.
Follow-up question: If you would not initiate therapy, would you perform a follow-up MRI?
Among the respondents who would not initiate therapy based on this case scenario, the majority (57%) would not perform a follow-up MRI. Respondents who would perform a follow-up MRI listed 3–6 months (25%), 6–12 months (40%), or 12–18 months (35%) as preferred time frames for re-evaluation of this aspect of disease activity.
Follow-up question: Would the presence of one or more Gd+ brain and/or spinal cord lesions in this patient influence your decision to initiate treatment with a DMT for this patient?
There was consensus (85%) to initiate treatment with a DMT if gadolinium-enhanced (Gd+) lesions were detected on the initial MRI. However, as with T2 lesions, there was no consensus on initial therapy choice (table 3, right column). Responses under the “other” category again listed chemotherapy or immunosuppressant drugs as preferred initial therapies.
DISCUSSION
Approximately 50% of patients with RRMS will eventually develop SPMS, characterized by increasing, persistent disability progression and infrequent or no new clinical attacks (SPMS).3 The timing and severity of transition to SPMS is highly variable, resulting in difficulties with both accurate long-term prognosis and effective intervention.1 Likewise, the gradual progression of disability without superimposed relapses experienced by patients with PPMS can be challenging to diagnose and also difficult to treat with currently available therapies. Indeed, none of the currently available DMTs has demonstrated any consistent benefit or clinical advantage in patients with SPMS or PPMS. The prognosis for patients with PPMS is highly variable, but life expectancy after diagnose has been estimated at 25 years.1 Optic neuritis, brainstem dysfunction, incomplete transverse myelitis, and difficulties with gait and coordination have been associated with worse disease outcomes,1,15 although a meta-analysis found that optic neuritis at presentation was not a consistent predictor of patient prognosis.16
The timing and severity of transition to SPMS is highly variable, resulting in difficulties with both accurate long-term prognosis and effective intervention.
SPMS
The SPMS case presented described a patient on a DMT initially for RRMS who develops gradual disability progression over an extended time frame and MRI evidence of new T2 lesions. There was consensus among the survey respondents to change the patient's MS treatment. Currently no class I evidence indicates a clinical benefit for either initiating approved DMTs in SPMS or switching MS therapies with ongoing disability progression in SPMS. Although the introduction and widespread use of the first-line MS therapies IFNβ and GA have had a large positive impact on MS care, they have proven markedly less effective in treating progressive forms of MS. A recent analysis of published data17 showed that in clinical trials, IFNβ consistently reduced SPMS patient relapse rates and accumulation of new MRI lesions, but results were conflicting for time to disability progression. Current evidence suggests that IFNβ therapy may be more effective in the early stages of SPMS, characterized by relapsing episodes and MRI evidence of greater brain lesion disease activity.9,17 There are currently no data supporting the use of GA in the treatment of patients with SPMS.17,18
MRI is a useful, reliable tool for monitoring disease activity in RRMS. However, the number of T2 and Gd+ lesions increases more rapidly in RRMS than in SPMS, with weaker correlations between MRI lesions and disability in the later disease stages.19 The application of MRI to MS diagnosis and monitoring has evolved20 and now includes newer MRI measures of T1 hypointense lesions (“black holes”), brain atrophy, and composite scale scores that incorporate the results of different MRI lesion and atrophy measures.e1,e2 Ongoing improvements in MRI methodologies may yield MRI endpoints with high specificity and sensitivity for use as noninvasive clinical correlates predictive of patient risk for MS progression.
To assess the importance of ongoing disability progression in SPMS treatment decisions, survey respondents were asked whether clinical evidence of disability progression, such as changes in cognition, ambulation, gait, paresis, vision, bladder function, bowel function, or ability to complete activities of daily living, was sufficient evidence of treatment failure to prompt a DMT switch. The majority of respondents (60%) responded affirmatively, with changes in cognition, ambulation, and gait most often cited as important indicators of insufficient treatment efficacy and the need to switch MS therapies.
The majority of survey respondents who would switch therapies for a patient who developed SPMS with ongoing disability progression and new MRI T2 lesions selected natalizumab as the new therapy, with a minority of respondents selecting fingolimod or mitoxantrone. Natalizumab has been shown to be effective in reducing RRMS-associated relapses and disability progression, particularly in patients with aggressive disease.e3 It was also recently reported to reduce relapses in patients with SPMS, although there was no significant change in mean patient Expanded Disability Status Scale score, a measure of disability progression.e3 Although natalizumab has provided a highly effective treatment option, it also carries the risk of development of progressive multifocal leukoencephalopathy (PML), a rare, potentially lethal viral infection.e4 Natalizumab efficacy in reducing disability progression in approximately 856 subjects with SPMS and disease progression independent of relapses is currently being evaluated in an ongoing phase 3b, multicenter, international, randomized, double-blind, placebo-controlled trial (ClinicalTrials.gov identifier: NCT01416181).
Ongoing improvements in MRI methodologies may yield MRI endpoints with high specificity and sensitivity for use as noninvasive clinical correlates predictive of patient risk for MS progression.
The sphingosine-1-phosphate receptor modulator fingolimod, which was approved in September 2010 in the United States for the treatment of RRMS, was selected as a switching therapy by a minority of respondents. It is important to note that the current surveys were administered in January and February 2011, shortly after fingolimod marketing approval, which likely influenced participant responses pertaining to treatment preferences. Based on its ability to inhibit the migration of dendritic cells,e5 which are thought to play a promotional role in disability progression during progressive forms of MS, fingolimod could theoretically be beneficial in slowing disability progression in SPMS.
The chemotherapeutic drug mitoxantrone is approved in the United States for treating patients with SPMS and has been shown to slow MS disease progression in this population,e6 although its use is limited by potential risks such as cardiotoxicity and leukemia.6,e6,e7 A dose-reduced, long-term mitoxantrone regimen may represent a feasible alternative treatment for patients without any other therapeutic options.e8 However, the lifetime limit for total mitoxantrone dose should be considered when planning long-term disease management.
A number of approved and investigational drugs are currently undergoing clinical evaluation for efficacy in SPMS5 and are not reviewed here. The majority of current MS therapies target the inflammatory components of MS pathophysiology, which are dominant during early disease but may not be appropriate therapeutic targets for slowing or stopping disability progression. A greater understanding of MS neurodegenerative processes may facilitate the selection of appropriately targeted therapies.
PPMS
Patients with PPMS continue to present a special clinical challenge for neurologists. For the PPMS case, there was consensus among the survey respondents to perform a spinal cord MRI and LP, presumably to confirm the initial diagnosis. As presented, the patient's symptoms and diagnostic evaluations are in line with the current McDonald MS diagnostic criteria.14 If the spinal cord MRI results and CSF were consistent with MS, a majority of respondents would initiate treatment with a DMT, but there was no consensus on this point. Treatment choices were highly variable, with no one therapy being selected by the majority of respondents in any of case scenarios presented.
This lack of consensus likely reflects the lack of effective therapies for this form of MS.4 Evaluations of immunomodulatory and immunosuppressive drugs for the treatment of PPMS have all yielded disappointing results. Indeed, although the immunomodulatory drugs IFNβe9-e11 and GAe12 and the immunosuppressive drugs mitoxantrone,e13 rixutimab,e14 and cladribinee15 have showed some individual patient benefit, each of these drugs has been ineffective in reducing disability progression in the majority of patients with PPMS. Given these results, it was noteworthy that the majority of respondents would still initiate a DMT. Ongoing clinical trials5 of established and novel therapies for the treatment of PPMS may yet demonstrate positive patient outcomes.
The findings presented herein reflect current MS practice patterns for SPMS and PPMS according to US-based survey respondents. These findings may not be applicable in other countries on the basis of potential treatment access limitations, for example, limitations due to reimbursement restrictions in public payor programs. It has been well-documented that there is widespread variation in clinical practice.e16 The intent of this survey was to both highlight current points of consensus and note where further scientific and evidence-based data are needed. The lack of agreement among US MS experts on the best treatment approaches for SPMS or PPMS clearly highlights the need for effective therapies.
This study sought to gain an understanding of the current diagnostic and treatment practices of MS specialists at US MS treatment centers. Table 4 provides a summary of the points of clear consensus among the survey participants on the treatment of SPMS and PPMS. With the evolving MS treatment landscape and the rapid evolution of diagnostic and prognostic indicators for this disease, there may be utility in periodically reassessing real-world clinical practice to better understand and to improve patient care.
Table 4.
Summary of consensus points
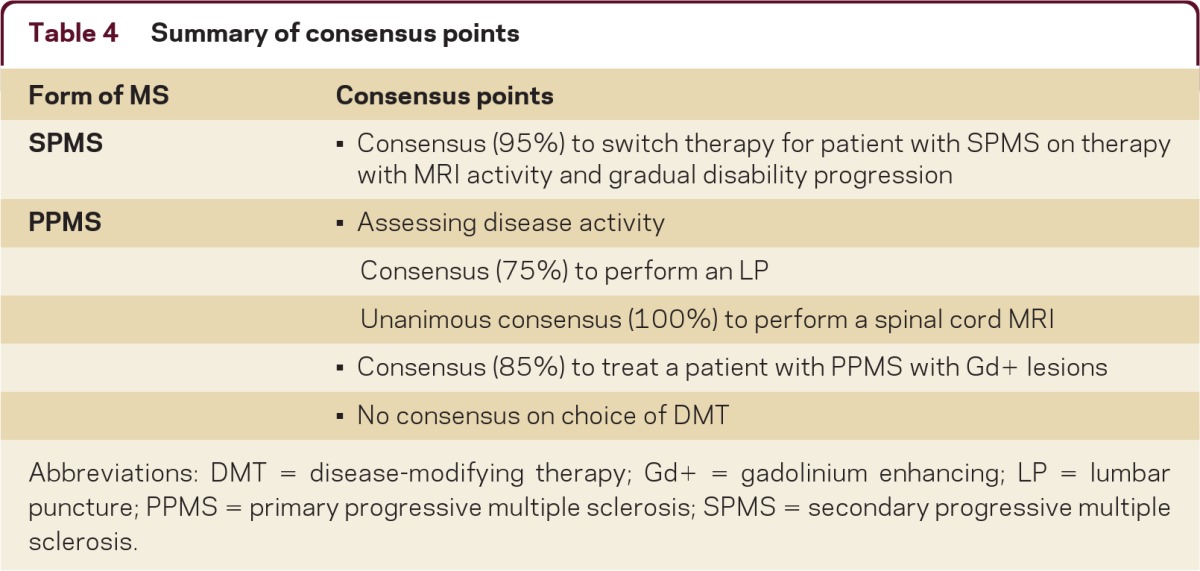
Abbreviations: DMT = disease-modifying therapy; Gd+ = gadolinium enhancing; LP = lumbar puncture; PPMS = primary progressive multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the individuals who took time to participate in this survey; Rohit Bakshi, MD, and Mariko Kita, MD, for their contributions to and support of this study; and Joshua Safran and Jacqueline Cannon with Infusion Communications for copyediting the manuscript.
Footnotes
Supplemental data www.neurology.org/cp
STUDY FUNDING
Biogen Idec convened a Steering Committee to develop the content of this article and paid committee members for their participation. In addition, Biogen Idec paid honoraria for survey participation and provided funding for study logistics and editorial support. Biogen Idec reviewed drafts and had input into the development of the surveys, reviewed the manuscript, and provided feedback on the manuscript to the authors. The authors had full editorial control of the surveys and manuscript and provided their final approval of all content.
DISCLOSURES
Dr. Khan has received funding for travel or speaker honoraria from Teva Pharmaceutical Industries Ltd., Novartis, and Biogen Idec; serves as a consultant for Teva Pharmaceutical Industries Ltd., Novartis, Biogen Idec, Merck Serono, Roche, and Genzyme Corporation; serves on speakers' bureaus for Teva Pharmaceutical Industries Ltd., Novartis, Genzyme Corporation, and Biogen Idec; and receives research support from Teva Pharmaceutical Industries Ltd., Novartis, Biogen Idec, Genzyme Corporation, Roche, the NIH, and the National MS Society. Dr. Miller serves on scientific advisory boards for sanofi-aventis, Biogen Idec, GlaxoSmithKline, EMD Serono, Teva Pharmaceutical Industries Ltd., Daiichi Sankyo, Merck Serono, Novartic, Ono Pharmaceutical Co. Ltd., and Acorda Therapeutics; serves as a consultant for Acorda Therapeutics, Biogen Idec, CVS Caremark, GlaxoSmithKline, and Novartis; serves on the editorial board of Continuum; has received speaker honoraria from Merck Serono and Teva Pharmaceutical Industries Ltd.; receives/has received research support from Acorda Therapeutics, Novartis, Genzyme Corporation, sanofi-aventis, and Biogen Idec; and has reviewed medico-legal cases as a defense expert. Dr. Tornatore serves on speakers' bureaus for Biogen Idec and Teva Pharmaceutical Industries Ltd.; serves on scientific advisory boards for Biogen Idec and Novartis; and receives research support from Biogen Idec. Dr. Phillips serves as a consultant and on scientific advisory boards for and has received speaker honoraria from Acorda Therapeutics Inc., Biogen Idec, Genzyme Corporation, Novartis, and Teva Pharmaceutical Industries Ltd. Dr. Barnes is an employee of Infusion Communications, which received funding from Biogen Idec.
REFERENCES
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2002;359:1221–1231 [DOI] [PubMed] [Google Scholar]
- 2. Tremlett H, Zhao Y, Rieckmann P, Hutchinson M. New perspectives in the natural history of multiple sclerosis. Neurology 2010;74:2004–2015 [DOI] [PubMed] [Google Scholar]
- 3. Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron 2006;52:61–76 [DOI] [PubMed] [Google Scholar]
- 4. Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol 2007;6:903–912 [DOI] [PubMed] [Google Scholar]
- 5. Fitzner D, Simons M. Chronic progressive multiple sclerosis: pathogenesis of neurodegeneration and therapeutic strategies. Curr Neuropharmacol 2010;8:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kappos L, Polman C, Pozzilli C, Thompson A, Beckmann K, Dahlke F; European Study Group in Interferon beta-1b in Secondary-Progressive MS Final analysis of the European multicenter trial on IFNbeta-1b in secondary-progressive MS. Neurology 2001;57:1969–1975 [DOI] [PubMed] [Google Scholar]
- 7. Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon beta-1a on MSFC progression in secondary progressive MS. Neurology 2002;59:679–687 [DOI] [PubMed] [Google Scholar]
- 8. Smith B, Carson S, Fu R, et al. Drug class review: disease-modifying drugs for multiple sclerosis: final update 1 report [report on the Internet] [cited 2011 Aug 24]. In: Drug Class Reviews. Portland, OR: Oregon Health & Science University; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK50570/pdf/TOC.pdf [PubMed] [Google Scholar]
- 9. Nikfar S, Rahimi R, Abdollahi M. A meta-analysis of the efficacy and tolerability of interferon-β in multiple sclerosis, overall and by drug and disease type. Clin Ther 2010;32:1871–1888 [DOI] [PubMed] [Google Scholar]
- 10. Goodin DS, Cohen BA, O'Connor P, Kappos L, Stevens JC; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology Assessment: the use of natalizumab (Tysabri) for the treatment of multiple sclerosis (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008;71:766–773 [DOI] [PubMed] [Google Scholar]
- 11. Kappos L, Radue E, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401 [DOI] [PubMed] [Google Scholar]
- 12. Marriott JJ, Miyasaki JM, Gronseth G, O'Connor PW; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology Evidence report: the efficacy and safety of mitoxantrone (Novantrone) in the treatment of multiple sclerosis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;74:1463–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krieger S. Multiple sclerosis therapeutic pipeline: opportunities and challenges. Mt Sinai J Med 2011;78:192–206 [DOI] [PubMed] [Google Scholar]
- 14. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barten LJ, Allington DR, Procacci KA, Rivey MP. New approaches in the management of multiple sclerosis. Drug Des Dev Ther 2010;4:343–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langer-Gould A, Popat RA, Huang SM, et al. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: a systematic review. Arch Neurol 2006;63:1686–1691 [DOI] [PubMed] [Google Scholar]
- 17. Bates D. Treatment effects of immunomodulatory therapies at different stages of multiple sclerosis in short-term trials. Neurology 2011;76(suppl 1):S14–S25 [DOI] [PubMed] [Google Scholar]
- 18. Ford C, Goodman AD, Johnson K, et al. Continuous long-term immunomodulatory therapy in relapsing multiple sclerosis: results from the 15-year analysis of the US prospective open-label study of glatiramer acetate. Mult Scler 2010;16:342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li DK, Held U, Petkau J, et al. ; Sylvia Lawry Centre for MS Research MRI T2 lesion burden in multiple sclerosis: a plateauing relationship with clinical disability. Neurology 2006;66:1384–1389 [DOI] [PubMed] [Google Scholar]
- 20. Lövblad KO, Anzalone N, Dörfler A, et al. MR imaging in multiple sclerosis: review and recommendations for current practice. Am J Neuroradiol 2010;31:983–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



