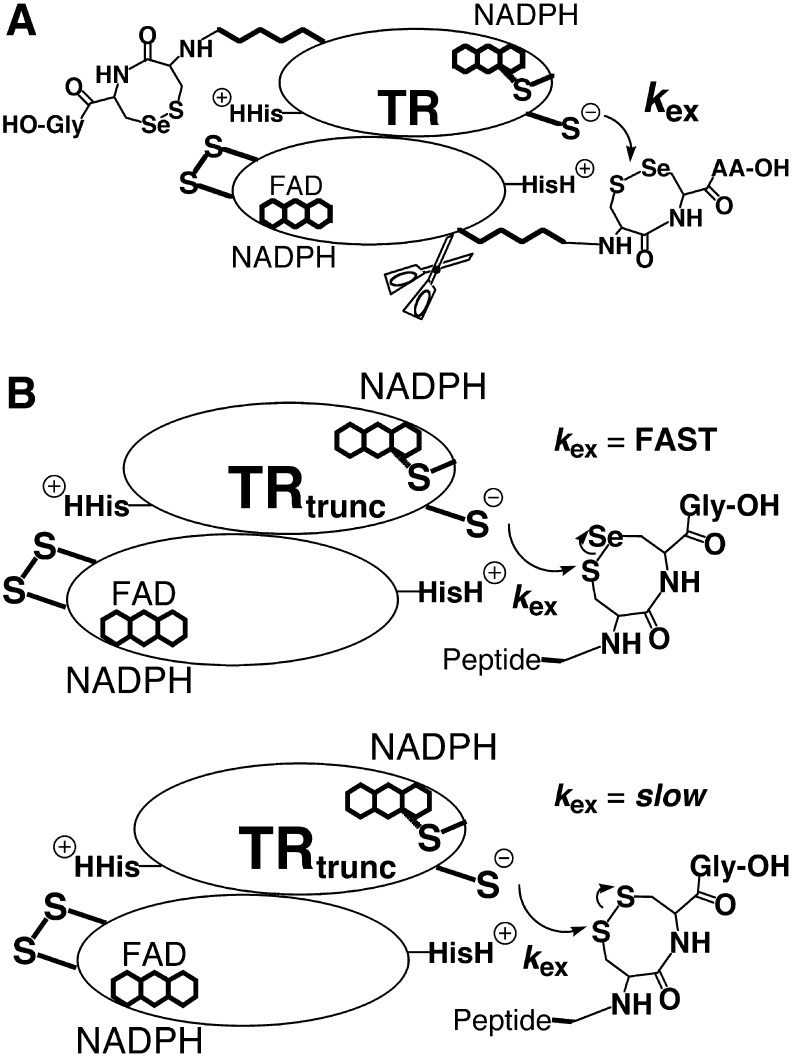
FIG. 4.
Method for analyzing the exchange step in the TR reaction mechanism. (A) A truncated TR was constructed by making a deletion mutant missing the last eight amino acids of the flexible tail containing the C-terminal redox center. (B) Synthetic peptides were constructed comprised of the eight missing amino acids. The advantage of this method is that the exchange step between N- and C-terminal redox centers could be isolated from the rest of the enzymatic reaction mechanism. In addition, the redox state of the peptide can be controlled so that all of the peptide is in the oxidized form, which is the substrate for the N-terminal redox center. The original interpretation of this data was that selenium was acting as a leaving group in the exchange step as shown here.