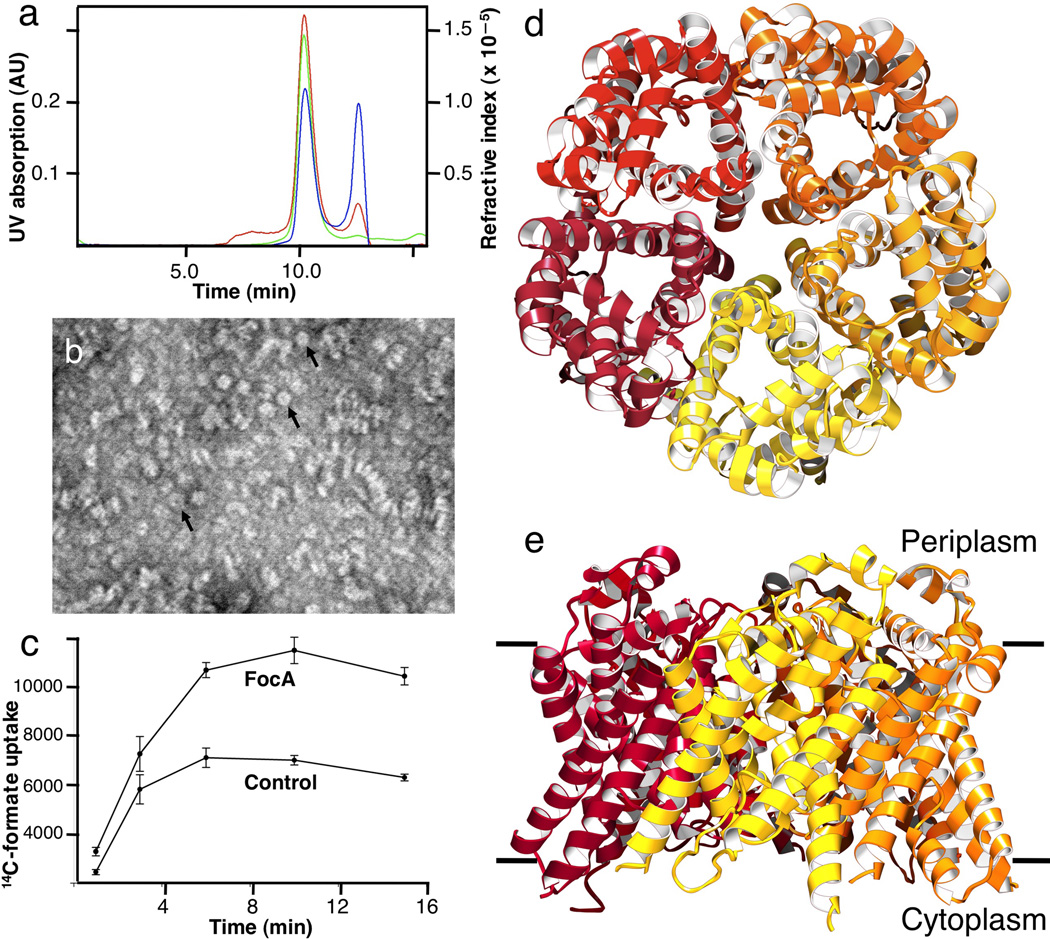
Figure 1.
Characterization of the FocA protein from Vibrio Cholerae. (a) Measurements of protein mass in solution. The protein has an apparent molecular weight of 303 kDa, which includes 142 kDa of bound octylglucoside detergent and phospholipid, indicating a 161 kDa FocA pentamer. The green line indicates UV absorbance at 280 nm, the red line static light scattering, and the blue line differential refractive index measurements. (b) Electron micrograph of FocA showing purified protein in various orientations. A few ring-like particles with a central cavity, probably representing a pentamer, are indicated by arrowheads. The scale bar represents 500 Å. (b) Uptake of 14C-labeled formate into proteoliposomes reconstituted from purified FocA at pH 6.7. Liposomes devoid of protein (Control) showed marked permeability to formate, as expected for a monovalent substrate with a pKa of 3.8. (d) Pentameric structure of FocA from the 2.13 Å resolution crystal structure, viewed from the periplasm. (e) FocA pentamer viewed from within the membrane plane.