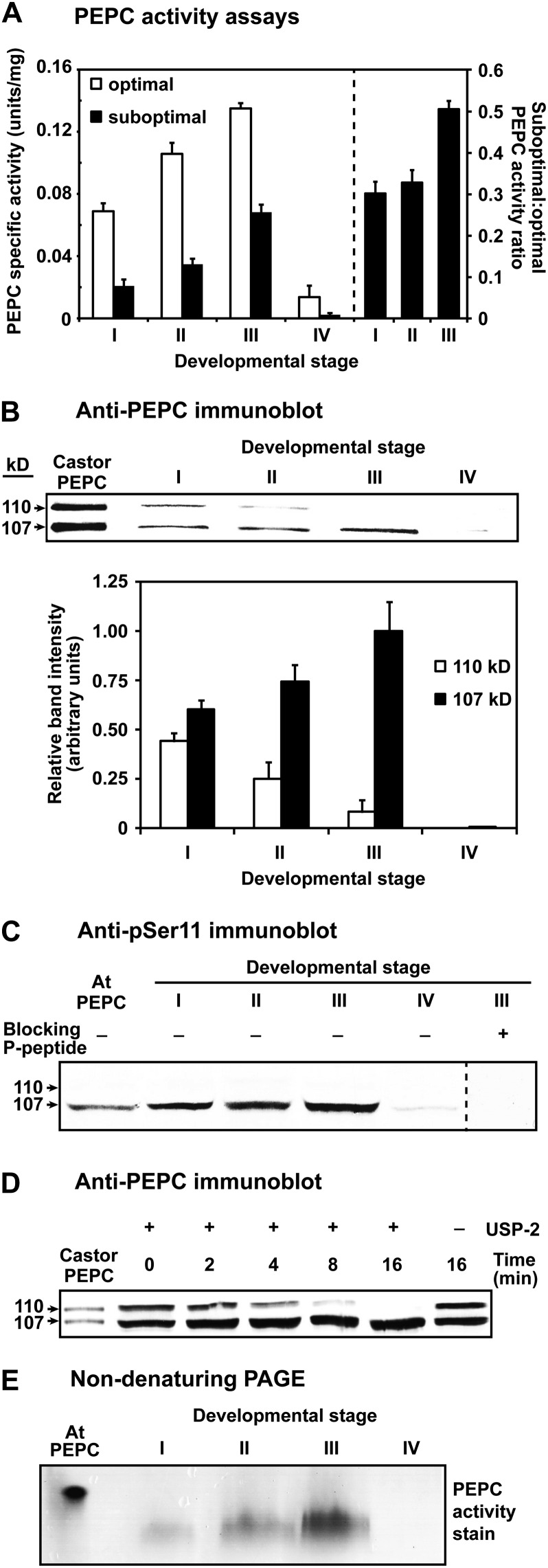
Figure 2.
Influence of proteoid root development on PEPC activity, subunit composition, and PTMs. A, All values represent the mean (±se) PEPC-specific activity of duplicate determinations of desalted extracts from n = four biological replicates determined under optimal (pH 8.2, 2.5 mm PEP) or suboptimal (pH 7.2, 0.2 mm PEP, 0.125 mm malate) conditions. All differences in specific activity and suboptimal:optimal activity ratio between stage I and III roots were statistically significant (P < 0.05). B, Hakea extracts were subjected to SDS-PAGE (10% gels; 0.5 μg protein lane−1), electroblotted onto a poly(vinylidene difluoride) membrane, and probed with anti-PEPC. Relative band intensity (±se) was quantified on n = five biological replicates from scanned immunoblots using ImageJ. C, Immunoblots were probed with anti-pSer11 in the presence of 10 μg mL−1 of the corresponding dephosphopeptide or phosphopeptide (20 μg of protein lane−1 for hakea extracts). D, Desalted extracts of stage I roots were incubated with and without 15 μΜ USP-2 and then immunoblotted with anti-PEPC (2 μg protein lane−1). E, In-gel PEPC activity staining followed nondenaturing PAGE of proteoid root extracts (7.5 μg protein lane−1) on 7% gels. The lanes labeled “Castor PEPC” (B and D) contained 50 and 25 ng, respectively, of purified monoubiquitinated Class-1 PEPC (RcPPC3) from germinating COS (Uhrig et al., 2008). The lanes labeled “At PEPC” (C and E) contained 250 and 500 ng, respectively, of purified phosphorylated Class-1 PEPC (AtPPC1) from –Pi Arabidopsis suspension cells (Gregory et al., 2009).