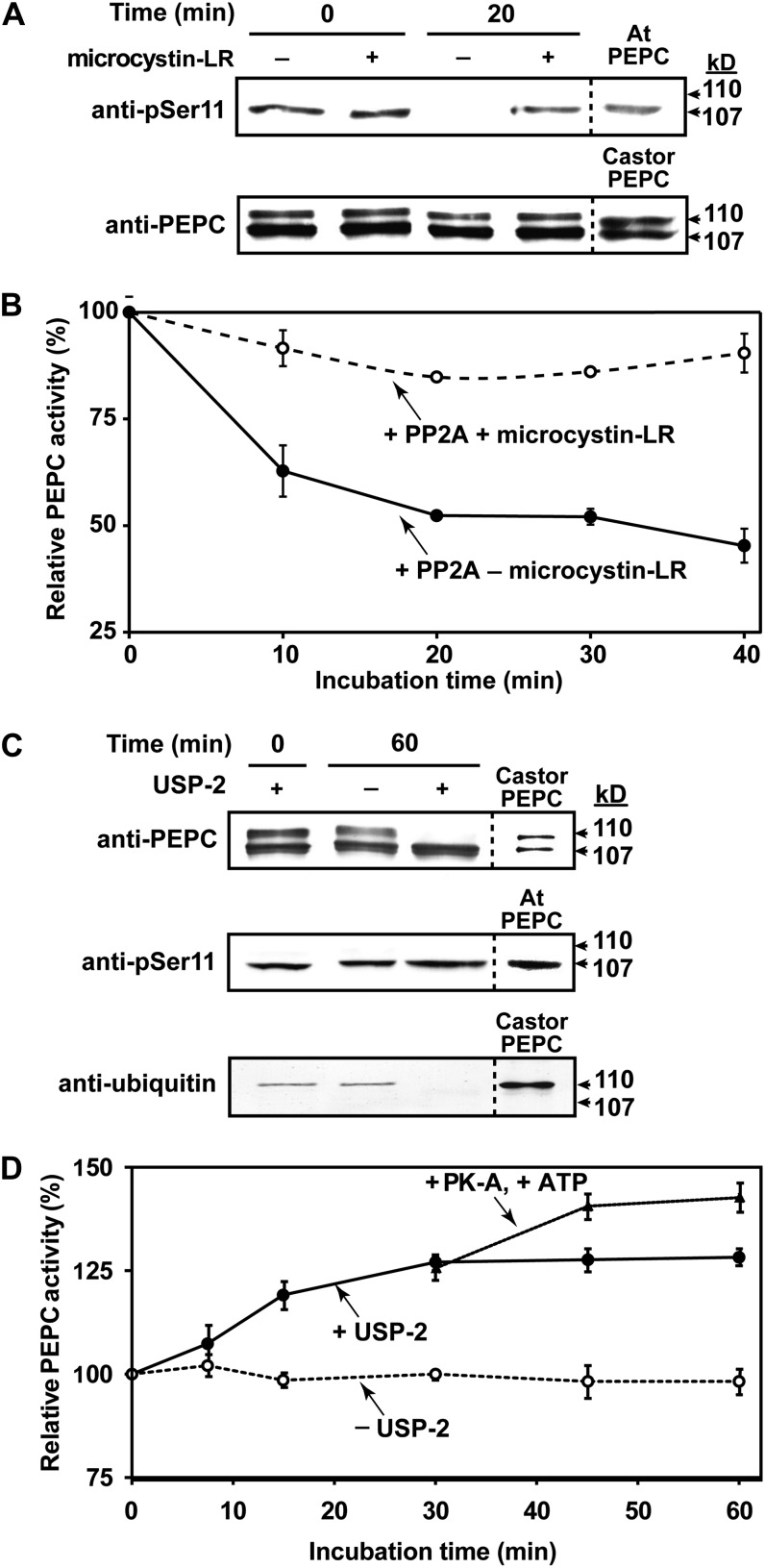
Figure 6.
Influence of in vitro dephosphorylation by PP2A (A and B) or deubiquitination by USP-2 (C and D) on activity of PEPC purified from stage I and II proteoid roots. A, Immunoblot analysis was performed using anti-PEPC or anti-pSer11 (75 ng or 1 μg of hakea PEPC lane−1, respectively. “Castor PEPC” denotes 50 ng of monoubiquitinated PEPC (RcPPC3) purified from germinating COS (Uhrig et al., 2008), whereas “At PEPC” denotes 500 ng of phosphorylated-PEPC (AtPPC1) purified from –Pi Arabidopsis (Gregory et al., 2009). B, Time course for PP2A-dependent inhibition of hakea PEPC activity (±50 nm microcystin-LR). C, Immunoblot analysis was performed using anti-PEPC, anti-pSer11, or antiubiquitin (75 ng, 1 μg, or 4 μg of hakea PEPC lane−1, respectively; reference lanes contained 50 ng of germinating COS PEPC, 0.5 μg of –Pi Arabidopsis PEPC, or 1 μg of germinating COS PEPC, respectively). D, Influence of USP-2 mediated deubiquitination, followed by bovine heart protein kinase-A mediated phosphorylation after 30 min, on activity of hakea PEPC. PEPC was assayed using suboptimal conditions (pH 7.2, 0.2 mm PEP, 0.125 mm malate). All values represent the mean (±se) of n = three independent experiments.