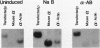Abstract
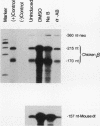
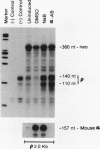
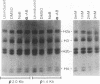
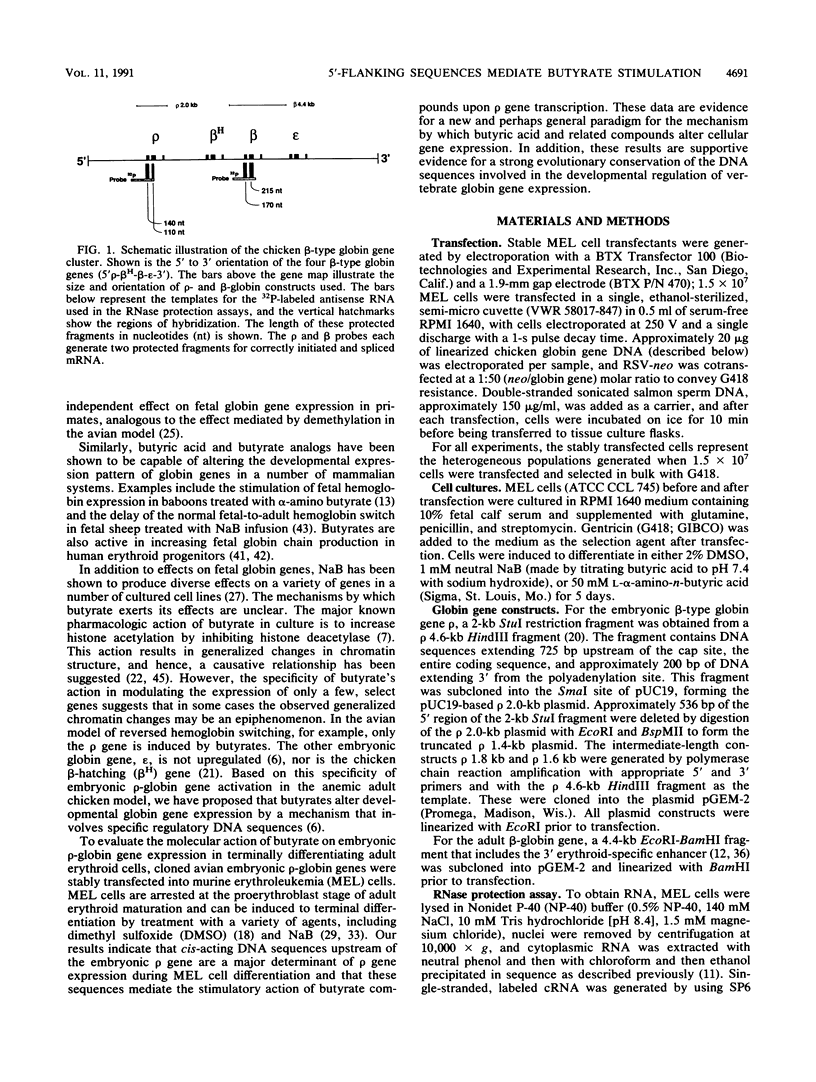
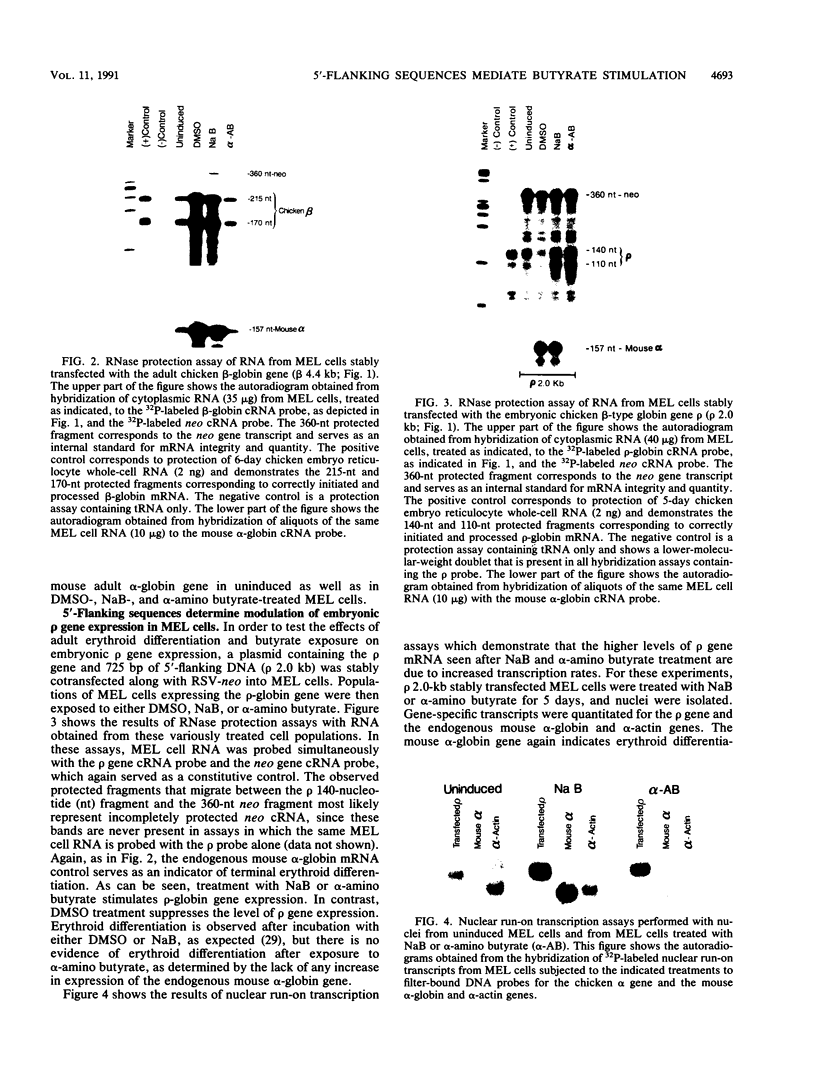
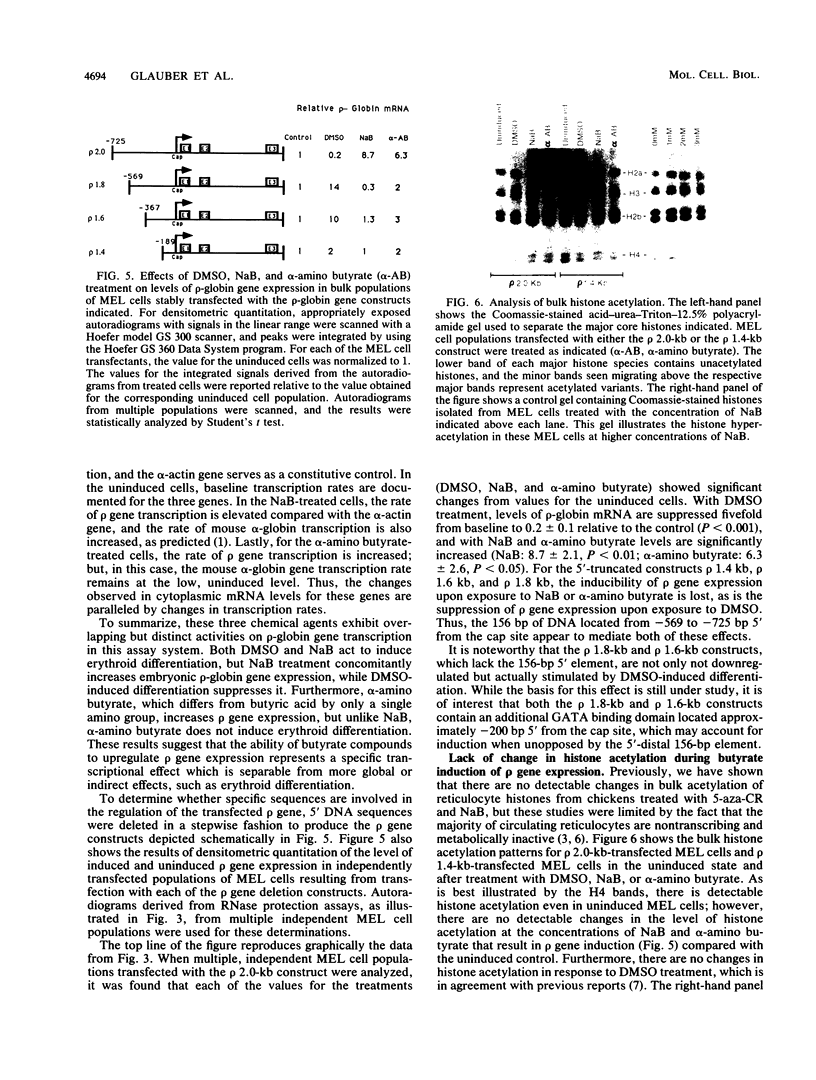
A stable transfection assay was used to test the mechanism by which embryonic globin gene transcription is stimulated in adult erythroid cells exposed to butyric acid and its analogs. To test the appropriate expression and inducibility of chicken globin genes in murine erythroleukemia (MEL) cells, an adult chicken beta-globin gene construct was stably transfected. The chicken beta-globin gene was found to be coregulated with the endogenous adult mouse alpha-globin gene following induction of erythroid differentiation of the transfected MEL cells by incubation with either 2% dimethyl sulfoxide (DMSO) or 1 mM sodium butyrate (NaB). In contrast, a stably transfected embryonic chicken beta-type globin gene, rho, was downregulated during DMSO-induced MEL cell differentiation. However, incubation with NaB, which induces MEL cell differentiation, or alpha-amino butyrate, which does not induce differentiation of MEL cells, resulted in markedly increased levels of transcription from the stably transfected rho gene. Analysis of histone modification showed that induction of rho gene expression was not correlated with increased bulk histone acetylation. A region of 5'-flanking sequence extending from -569 to -725 bp upstream of the rho gene cap site was found to be required for both downregulation of rho gene expression during DMSO-induced differentiation and upregulation by treatment with NaB or alpha-amino butyrate. These data are support for a novel mechanism by which butyrate compounds can alter cellular gene expression through specific DNA sequences. The results reported here are also evidence that 5'-flanking sequences are involved in the suppression of embryonic globin gene expression in terminally differentiated adult erythroid cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beru N., Maples P. B., Hermine O., Goldwasser E. Differential expression of alpha- and beta-globin genes in erythroleukemic cell lines. Mol Cell Biol. 1990 Jul;10(7):3591–3595. doi: 10.1128/mcb.10.7.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohan C. A., Robinson R. A., Luciw P. A., Srinivasan A. Mutational analysis of sodium butyrate inducible elements in the human immunodeficiency virus type I long terminal repeat. Virology. 1989 Oct;172(2):573–583. doi: 10.1016/0042-6822(89)90200-6. [DOI] [PubMed] [Google Scholar]
- Brotherton T. W., Covault J., Shires A., Chalkley R. Only a small fraction of avian erythrocyte histone is involved in ongoing acetylation. Nucleic Acids Res. 1981 Oct 10;9(19):5061–5073. doi: 10.1093/nar/9.19.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown B. A., Padgett R. W., Hardies S. C., Hutchison C. A., 3rd, Edgell M. H. beta-globin transcript found in induced murine erythroleukemia cells is homologous to the beta h0 and beta h1 genes. Proc Natl Acad Sci U S A. 1982 May;79(9):2753–2757. doi: 10.1073/pnas.79.9.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Ingram V. M. Structural studies on chick embryonic hemoglobins. J Biol Chem. 1974 Jun 25;249(12):3960–3972. [PubMed] [Google Scholar]
- Burns L. J., Glauber J. G., Ginder G. D. Butyrate induces selective transcriptional activation of a hypomethylated embryonic globin gene in adult erythroid cells. Blood. 1988 Nov;72(5):1536–1542. [PubMed] [Google Scholar]
- Candido E. P., Reeves R., Davie J. R. Sodium butyrate inhibits histone deacetylation in cultured cells. Cell. 1978 May;14(1):105–113. doi: 10.1016/0092-8674(78)90305-7. [DOI] [PubMed] [Google Scholar]
- Cao S. X., Gutman P. D., Dave H. P., Schechter A. N. Identification of a transcriptional silencer in the 5'-flanking region of the human epsilon-globin gene. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5306–5309. doi: 10.1073/pnas.86.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnay P., Treisman R., Mellon P., Chao M., Axel R., Maniatis T. Differences in human alpha- and beta-globin gene expression in mouse erythroleukemia cells: the role of intragenic sequences. Cell. 1984 Aug;38(1):251–263. doi: 10.1016/0092-8674(84)90547-6. [DOI] [PubMed] [Google Scholar]
- Chen E., Karr R. W., Frost J. P., Gonwa T. A., Ginder G. D. Gamma interferon and 5-azacytidine cause transcriptional elevation of class I major histocompatibility complex gene expression in K562 leukemia cells in the absence of differentiation. Mol Cell Biol. 1986 May;6(5):1698–1705. doi: 10.1128/mcb.6.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Constantoulakis P., Papayannopoulou T., Stamatoyannopoulos G. alpha-Amino-N-butyric acid stimulates fetal hemoglobin in the adult. Blood. 1988 Dec;72(6):1961–1967. [PubMed] [Google Scholar]
- Covault J., Chalkley R. The identification of distinct populations of acetylated histone. J Biol Chem. 1980 Oct 10;255(19):9110–9116. [PubMed] [Google Scholar]
- Cowie A., Myers R. M. DNA sequences involved in transcriptional regulation of the mouse beta-globin promoter in murine erythroleukemia cells. Mol Cell Biol. 1988 Aug;8(8):3122–3128. doi: 10.1128/mcb.8.8.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly S., Skoultchi A. I. Absolute rates of globin gene transcription and mRNA formation during differentiation of cultured mouse erythroleukemia cells. J Biol Chem. 1985 Oct 5;260(22):12167–12173. [PubMed] [Google Scholar]
- Ginder G. D., Whitters M. J., Pohlman J. K. Activation of a chicken embryonic globin gene in adult erythroid cells by 5-azacytidine and sodium butyrate. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3954–3958. doi: 10.1073/pnas.81.13.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Dover G., Young N. S., Moore J. G., Charache S., Ley T., Nienhuis A. W. 5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J Clin Invest. 1985 Feb;75(2):547–557. doi: 10.1172/JCI111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Aida M., Inoue Y. Isolation and characterization of high and low differentiation-inducible Friend leukemia lines. Gan. 1976 Oct;67(5):767–770. [PubMed] [Google Scholar]
- Kruh J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol Cell Biochem. 1982 Feb 5;42(2):65–82. doi: 10.1007/BF00222695. [DOI] [PubMed] [Google Scholar]
- Landes G. M., Martinson H. G. Transcriptional properties of chick embryonic erythroid nuclei in vitro. J Biol Chem. 1982 Sep 25;257(18):11002–11007. [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Nathan D. G. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Anagnou N. P., Keller G. H., Humphries R. K., Turner P. H., Young N. S., Keller P., Nienhuis A. W. 5-azacytidine selectively increases gamma-globin synthesis in a patient with beta+ thalassemia. N Engl J Med. 1982 Dec 9;307(24):1469–1475. doi: 10.1056/NEJM198212093072401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Noguchi C. T., Turner P. H., Schechter A. N., Heller P., Nienhuis A. W. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickol J. M., Felsenfeld G. Bidirectional control of the chicken beta- and epsilon-globin genes by a shared enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2548–2552. doi: 10.1073/pnas.85.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T. H., Brice M., Stamatoyannopoulos G. Stimulation of fetal hemoglobin synthesis in bone marrow cultures from adult individuals. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2033–2037. doi: 10.1073/pnas.73.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Torrealba de Ron A., Veith R., Knitter G., Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Vichinsky E., Stamatoyannopoulos G. Fetal Hb production during acute erythroid expansion. I. Observations in patients with transient erythroblastopenia and post-phlebotomy. Br J Haematol. 1980 Apr;44(4):535–546. doi: 10.1111/j.1365-2141.1980.tb08707.x. [DOI] [PubMed] [Google Scholar]
- Perrine S. P., Miller B. A., Faller D. V., Cohen R. A., Vichinsky E. P., Hurst D., Lubin B. H., Papayannopoulou T. Sodium butyrate enhances fetal globin gene expression in erythroid progenitors of patients with Hb SS and beta thalassemia. Blood. 1989 Jul;74(1):454–459. [PubMed] [Google Scholar]
- Perrine S. P., Miller B. A., Greene M. F., Cohen R. A., Cook N., Shackleton C., Faller D. V. Butryic acid analogues augment gamma globin gene expression in neonatal erythroid progenitors. Biochem Biophys Res Commun. 1987 Oct 29;148(2):694–700. doi: 10.1016/0006-291x(87)90932-6. [DOI] [PubMed] [Google Scholar]
- Perrine S. P., Rudolph A., Faller D. V., Roman C., Cohen R. A., Chen S. J., Kan Y. W. Butyrate infusions in the ovine fetus delay the biologic clock for globin gene switching. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8540–8542. doi: 10.1073/pnas.85.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raich N., Enver T., Nakamoto B., Josephson B., Papayannopoulou T., Stamatoyannopoulos G. Autonomous developmental control of human embryonic globin gene switching in transgenic mice. Science. 1990 Nov 23;250(4984):1147–1149. doi: 10.1126/science.2251502. [DOI] [PubMed] [Google Scholar]
- Reeves R., Gorman C. M., Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985 May 24;13(10):3599–3615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. G., Whittaker R. G., Neumann J. R., Ingram V. M. n-Butyrate causes histone modification in HeLa and Friend erythroleukaemia cells. Nature. 1977 Aug 4;268(5619):462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- Rodgers G. P., Dover G. J., Noguchi C. T., Schechter A. N., Nienhuis A. W. Hematologic responses of patients with sickle cell disease to treatment with hydroxyurea. N Engl J Med. 1990 Apr 12;322(15):1037–1045. doi: 10.1056/NEJM199004123221504. [DOI] [PubMed] [Google Scholar]
- Sheffery M., Marks P. A., Rifkind R. A. Gene expression in murine erythroleukemia cells. Transcriptional control and chromatin structure of the alpha 1-globin gene. J Mol Biol. 1984 Feb 5;172(4):417–436. doi: 10.1016/s0022-2836(84)80015-7. [DOI] [PubMed] [Google Scholar]
- Stuve L. L., Myers R. M. A directly repeated sequence in the beta-globin promoter regulates transcription in murine erythroleukemia cells. Mol Cell Biol. 1990 Mar;10(3):972–981. doi: 10.1128/mcb.10.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Veith R., Galanello R., Papayannopoulou T., Stamatoyannopoulos G. Stimulation of F-cell production in patients with sickle-cell anemia treated with cytarabine or hydroxyurea. N Engl J Med. 1985 Dec 19;313(25):1571–1575. doi: 10.1056/NEJM198512193132503. [DOI] [PubMed] [Google Scholar]
- Zhang J. W., Raich N., Enver T., Anagnou N. P., Stamatoyannopoulos G. Butyrate induces expression of transfected human fetal and endogenous mouse embryonic globin genes in GM 979 erythroleukemia cells. Dev Genet. 1990;11(2):168–174. doi: 10.1002/dvg.1020110207. [DOI] [PubMed] [Google Scholar]