Figure 7.
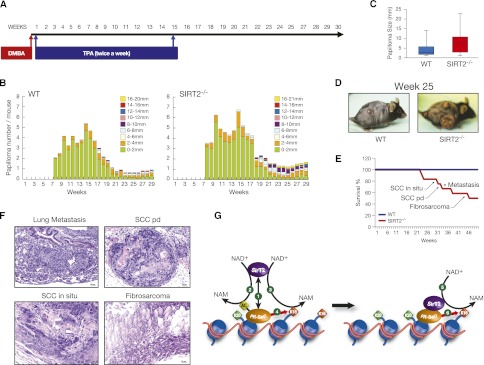
SIRT2−/− mice are more prone to tumorigenesis. (A) Schematic representation of the DMBA/TPA-induced skin tumorigenesis assay. (B) Average number of papillomas per mouse at the indicated number of weeks after starting the DMBA/TPA treatment. The treatment was interrupted at week 15. (C) Papilloma size in wild-type (WT) versus SIRT2−/− mice at week 25 after starting the treatment. (D) Representative wild-type and SIRT2−/− animals in week 25. (E) Survival curve of DMBA/TPA-treated wild-type and SIRT2−/− mice. (F) Representative examples of the neoplasias and metastases indicated in E developed by SIRT2−/− mice. (Top left image) Lung metastasis of a poorly differentiated skin squamous cell carcinoma. (Top right) Poorly differentiated skin squamous cell carcinoma (SCC pd) with pleomorphism and numerous mitotic cells, clearly growing invasively into the dermis, subcutaneous tissue, and subcutaneous muscle. (Bottom left) In situ skin squamous cell carcinoma (SCC in situ) with focal basal membrane disruption. (Bottom right) Cutaneous fibrosarcoma with pleomorphic spindle cells that invade skeletal muscle. (G) Proposed model of the regulation of H4K20me1 deposition by SirT2 based in our results. (1) PR-Set7 recruits SirT2 to specific chromatin regions. SirT2 then deacetylates both PRSet7 (2) and H4K16Ac (3) from neighboring nucleosomes. PR-Set7 deacetylation in K90 induces its mobilization. (4) Then, SirT2-bound PR-Set7 monomethylates H4K20 in the neighbor nucleosome. This is followed by translocation to the following nucleosome, where deacetylation of H4K16Ac (5) and H4K20me1 (6) take place again. Overall, we propose that SirT2 regulates the activation and spreading of PR-Set7 in G2/M.
