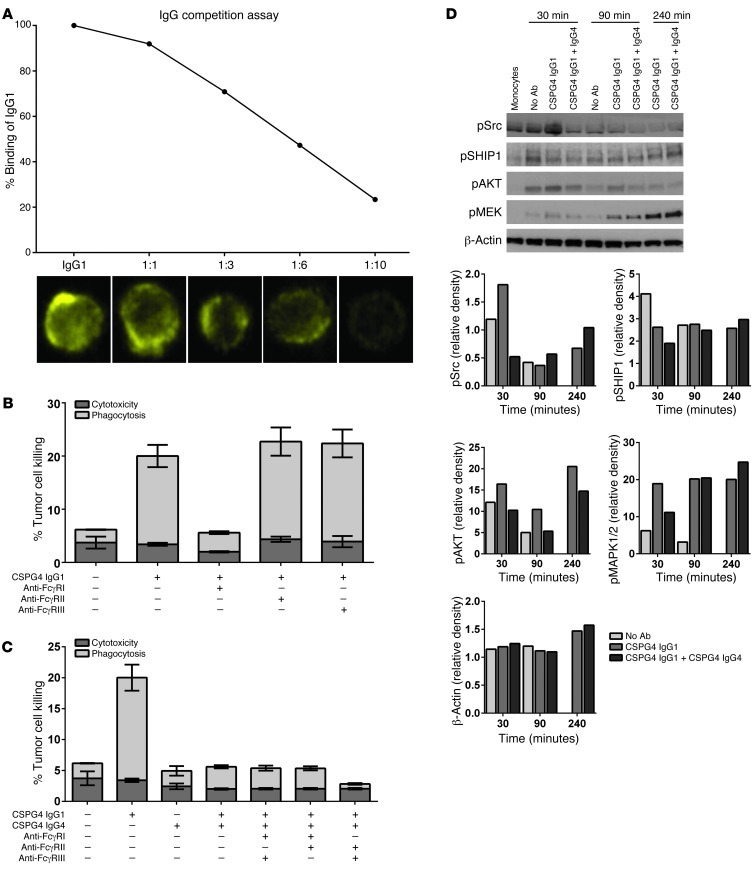
Figure 6. IgG4 blocks IgG1 antibody-dependent tumor cell killing by inhibiting IgG1 binding and activation through FcγRI.
(A) Competition assay of IgG1 binding on the surface of monocytic cells displaced by addition of increasing concentrations of IgG4 antibody. Proportion of cells binding IgG1 is decreased with increasing concentrations of IgG4, demonstrated by flow cytometric evaluations and representative confocal images (yellow, by ImageStream). (B) Anti-CSPG-4 IgG1-mediated tumor cell killing (by flow cytometry) is inhibited by addition of an antibody known to block IgG Fc binding to FcγRI but not with addition of blocking antibodies to FcγRII or to FcγRIII. (C) Inhibitory functions of anti-CSPG-4 IgG4 are not lost by blocking FcγRII or FcγRIII with previously described specific FcγR blocking antibodies in flow cytometric antibody-dependent tumor cell killing assays. (D) Protein extracts of primary human monocytes isolated by flow cytometric sorting at different times during the antibody-mediated tumor cell killing assay were examined for phosphorylated products of the FcγR signaling pathway. Western blots of phospho-proteins and band density quantifications relative to freshly isolated monocytes demonstrate that IgG4 inhibits the activatory signaling cascades of FcγR (Src, AKT, MEK), while lack of pSHIP implies that FcγRII signaling is not involved in the IgG4 blockade. (B and C) Data are representative figures of 3 independent experiments.