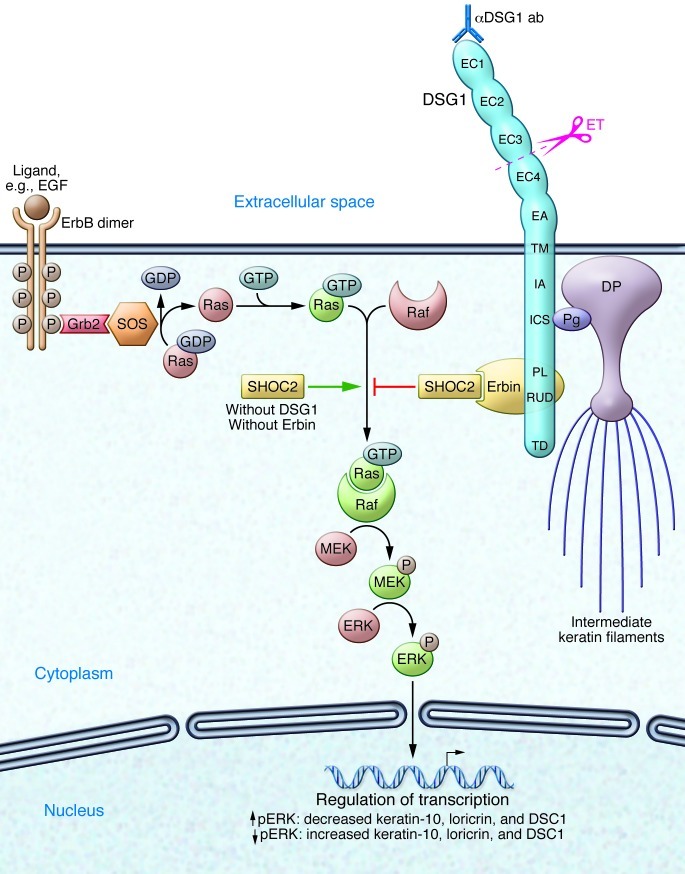
Figure 1. Keratinocyte structure, adhesion, and signaling modulated by Erbin and/or DSG1.
The ErbB family includes four receptors with tyrosine kinase activity (ErbB1 [also known as EGFR], ErbB2, ErbB3, ErbB4), which form homodimers and/or heterodimers upon ligand binding. Autophosphorylation leads to direct activation of the PI3K or phospholipase C-γ pathway (not shown). Via adaptor proteins (e.g., Grb2), the guanyl nucleotide exchange factor son of sevenless (SOS) allows exchange of GDP for GTP on Ras and thus activation of this small GTPase. The scaffolding protein SHOC2 accelerates formation of Ras/Raf complexes and leads, in absence of DSG1 and/or Erbin, to activation of the Raf/MEK/ERK pathway, which inhibits differentiation of keratinocytes. In the presence of DSG1, the scaffolding protein Erbin skews the fate of keratinocytes toward differentiation by binding SHOC2 and inhibiting formation of Ras/Raf complexes. Erbin binds to the cytoplasmic tail of DSG1 but not to the intracellular cadherin-like sequence (ICS) that binds plakoglobin (Pg), a protein important for desmosome integrity and function. ET from Staphylococcus aureus cleaves DSG1 between extracellular domain 3 (EC3) and EC4. Anti-DSG1 autoantibodies (αDSG1 ab) mediating PF predominantly target N-terminal extracellular domains, causing loss of cell-cell adhesion. DP, desmoplakin; EC1–EC4, extracellular domains of DSG1; EA, extracellular anchor (also known as EC5); TM, transmembrane domain; IA, intracellular anchor; PL, proline-rich linker; RUD, repeat unit domains; TD, terminal domain; DSC1, desmocollin-1.