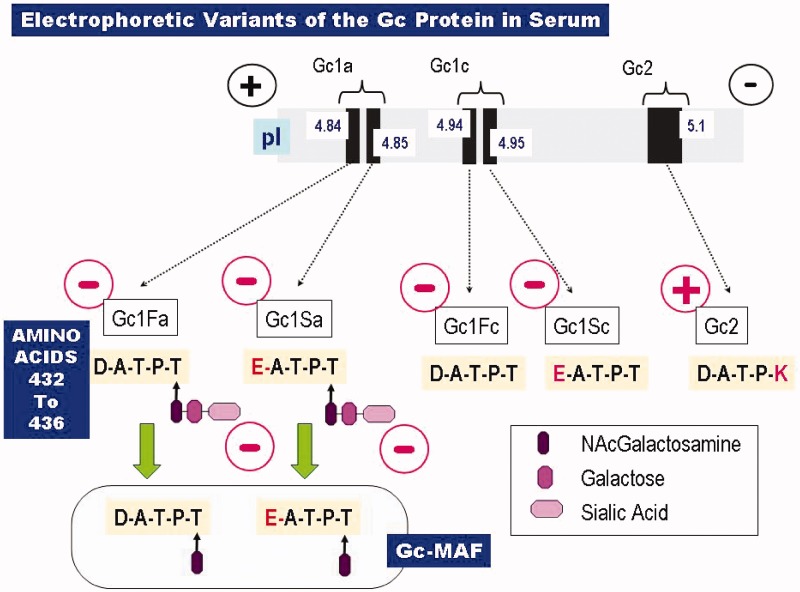
Figure 2.
Common electrophoretic variants of the Gc protein (DBP). Shown at the top is the relative electrophoretic separation of the various Gc species, based on their isoelectric points (pI). The corresponding molecular structures are given below that. Only five residues of primary amino acid sequence (residues 432–436 = Asp–Ala–Thr–Pro–Thr = D–A–T–P–T) are depicted, along with their O-linked saccharides. Note that the D432E mutation results in a very small separation (ΔpI = 0.01), since the carboxyl side chains of the wild-type aspartate residue (in the Gc1F species) and the mutant glutamate residue (in Gc1S) have such similar dissociation constants. There is larger separation of the two Gc1 species if the negatively charged sialic acid residue (generating the anodal form, Gc1a) is removed, generating Gc1c, the cathodal form. Gc-MAF, shown at the bottom, arises as a result of sequential deglycosylation removing first the sialic acid and then the galactose residues. At the T436 position, the genetic variant Gc2 shows a more marked cathodal shift in electrophoretic migration due to replacement of the O-glycosylation site by a positively charged lysine residue (436 K).