Abstract
In this study, the optimal conditions for the extraction of antioxidants from hard winter wheat bran (WH711) were determined using response surface methodology (RSM). A central composite face centred design was used to investigate the effects of three independent variables, namely solvent composition (%v/v), extraction temperature (°C) and time (minutes) on the responses; free phenolic content (FPC), total flavonoid content (TFC), total antioxidant activity (TAA), ferric reducing power (FRP), hydrogen peroxide scavenging activity (HPSA). Regression analysis showed that more than 90% of the variation was explained by the models of different responses. The optimal conditions for the antioxidantss extraction were found to be methanol concentration of 85%v/v, extraction temperature of 75 °C, extraction time of 45 min, for wheat bran. The experimental values of FPC, TFC, TAA, FRP and HPSA were 0.921 mg GAE/g bran (B), 0.4588 mg CE/g B, 0.01408 mM AAE/g B, 2.532 mM AAE/g B and 3.193 mM TE/g B, respectively which agreed with those predicted, thus indicating suitability of the model employed and the suitability of RSM in optimizing the extraction conditions.
Keywords: Wheat bran, Free phenolic compound (FPC), Total flavonoid compound (TFC), Total antioxidant activity (TAA), Response surface methodology (RSM), Extraction
Introduction
Plant and plant products are being used as a source of medicine since long time. The medicinal properties of plants have been investigated in the recent scientific developments throughout the world, due to their potent antioxidant activities, no side effects and economic viability (Kumar et al. 2007). Natural antioxidants or phytochemical are the secondary metabolites, of plants (Ramamoorthy and Bongo 2007) which are known as nutraceutical. Flavonoids and phenolic compounds widely distributed in plants which have been reported to exert multiple biological effect, including antioxidant, free radical scavenging abilities, anti-inflammatory, anticarcinogenic (Kumar et al. 2007).
Wheat (Triticum spp.) is one of the most important cereal grains which are physiologically composed of endosperm, bran and germ. Wheat bran is good source of secondary metabolites as phenolic acids, flavonoids, lignans, phytosterols, tocopherols and tocotrienols and dietary fiber.
Phenolic acids occur in a large number of plants as secondary metabolites. In case of plant foods, phenolics and polyphenolics constitute a main group of compounds that render beneficial effects, in part, due to their antioxidant potential, among other mechanisms of action. Phenolic acids are rather a small but important food component due to its antioxidative activity (Graf 1992). This basic property, which is very important for life, is associated with a number of biological functions such as antimutagenicity, anticarcinogenicity, and deceleration of the organism ageing. The antioxidative activity of cereal extracts is very different and depends on the extraction agent, the kind of cereals and, to a certain extent, also on the cultivar and the morphological fraction (Zielinski and Kozlowska 2000). Extract of wheat bran, having high concentration of phenolic acids, was shown to have stronger antioxidant activity than other fractions of wheat (Onyeneho and Hettiarachchy 1992). Ferulic acid is major phenolic acid present in wheat bran with other phenolic acid such as vanillic acid, p-coumaric acid, syringic acid, caffeic acid, p-hydroxybenzoic acid.
Significant levels of antioxidant activities were detected in wheat & wheat based food products suggesting that wheat may serve excellent dietary source of natural antioxidants for disease prevention & health promotion (Zhou et al. 2004). Carotenoids, flavonoids, cinnamic acids, benzoic acids, folic acid, ascorbic acid, tocopherols, tocotrienols are some of the antioxidants produced by the plant for their sustenance (Ramamoorthy and Bongo 2007).
In general, optimization of a process could be achieved by either empirical or statistical methods; the former having limitations toward complete optimization (Liyana-Pathirana and Shahidi 2005). Empirical method adopted one-factor-at-a-time approach which has inability to determine interaction between the variables, time consuming, costly and less effective (Chan et al. 2009). Response surface methodology (RSM) has been successfully used to model and optimize biochemical & biotechnological process related to food systems (Liyana-Pathirana and Shahidi 2005). The principles and foundations of RSM were first introduced by Box and Wilson. The main advantage of RSM enables evaluation of the effects of several process variables and their interactions on response variables. RSM is faster and more informative than the classical one-variable-at-a-time approach or the use of full factorial designs (Ozddemir et al. 2008).
Research shows that the interactions between genotype and growing conditions might significant alter the nutraceutical properties of the wheat bran. Many factors such as solvent composition, extraction time, extraction temperature, solvent to solid ratio, pH, and particle size, among others, may significantly influence the extraction efficacy (Kim et al. 2006; Qu et al. 2005; Marama et al. 2004; Pinzino 1999; Mpofu et al. 2006; Li et al. 2005; Silva et al. 2007). Many researchers studied different nutraceuticals present in different fraction of wheat under different conditions using methanol as solvent. The free phenolic content (FPC) (Kim et al. 2006) and lignans (Qu et al. 2005) in wheat bran; TPC, reducing power and DPPH in wheat and its fraction (Marama et al. 2004); carotenoid content in wheat grain (Pinzino 1999); DPPH in red and white wheat grain (Mpofu et al. 2006); TPC and DPPH in Chinese black grained wheat (Li et al. 2005) have been reported by the researchers.
The proper extraction of the active ingredient is essential if they are to be of prophylactic or therapeutic value in human subjects. Therefore, isolation, identification and quantification of phytochemicals in foods & evaluation of their potential health benefits are needed to study (Liyana-Pathirana and Shahidi 2005). Liyana-Pathirana and Shahidi (2005) already explored the optimization of extraction of the phenolic compounds present in the wheat by using ethanol/water. Absolute ethanol and 50%acetone have been used to prepare antioxidant extracts from wheat and wheat-based cereal products (Yu et al. 2002a, b), while 70% methanol and 50% acetone are widely accepted solvents for extracting phenolic compounds. Therefore, the present study deals with the extraction of antioxidants in particular to the Indian hard wheat variety (WH, 711) using methanol as an extraction solvent.
Materials & methods
Materials
Wheat grain sample of hard winter wheat variety (WH 711) were procured from local market of Rothak city, Haryana (northern part of India). Standard ascorbic acid and tocopherol were procured from Hi-Media, Bombay. Gallic Acid and catechin were procured from Sigma Aldrich, USA. All chemicals used in the study were either AR Grade or extra pure.
Experimental design and statistical analysis for antioxidants extraction
Response surface methodology (RSM) consists of a group of empirical techniques devoted to the evaluation of relation existing between a cluster of controlled experimental factors and the measured responses, according to one or more selected variables under investigation are necessary for achieving a more realistic model. Three variables were selected to find the optimized condition for antioxidants extraction using central composite face centred design (CCRD). CCRD provide relatively high quality predictions over the entire design space and do not require using points outside the original factor range. The independent variables studied were solvent concentration (% v/v), extraction temperature (°C), and extraction time (minutes) while response variables were free phenolic content, total flavonoids content, total antioxidant activity, hydrogen peroxide scavenging activity and reducing power. The range and the levels of the experiments variables used in the coded and uncoded form in this study are given in Table 1.
Table 1.
The experimental design in coded and uncoded form for the optimization of variables using central composite face centred design
| Standard ordera | Run Orderb | Coded | Uncoded | ||||
|---|---|---|---|---|---|---|---|
| Solvent Concentration (% v/v) | Temperature (°C) | Time (Minutes) | Solvent Concentration (% v/v) | Temperature (°C) | Time (Minutes) | ||
| 1 | 19 | −1 | −1 | −1 | 70 | 30 | 45 |
| 2 | 14 | 1 | −1 | −1 | 85 | 55 | 15 |
| 3 | 13 | −1 | 1 | −1 | 70 | 80 | 45 |
| 4 | 10 | 1 | 1 | −1 | 70 | 80 | 15 |
| 5 | 20 | −1 | −1 | 1 | 85 | 55 | 30 |
| 6 | 7 | 1 | −1 | 1 | 100 | 80 | 15 |
| 7 | 5 | −1 | 1 | 1 | 85 | 55 | 30 |
| 8 | 8 | 1 | 1 | 1 | 85 | 30 | 30 |
| 9 | 2 | −1 | 0 | 0 | 85 | 55 | 30 |
| 10 | 17 | 1 | 0 | 0 | 100 | 55 | 30 |
| 11 | 15 | 0 | −1 | 0 | 85 | 55 | 30 |
| 12 | 6 | 0 | 1 | 0 | 85 | 80 | 30 |
| 13 | 16 | 0 | 0 | −1 | 85 | 55 | 45 |
| 14 | 3 | 0 | 0 | 1 | 85 | 55 | 30 |
| 15 | 4 | 0 | 0 | 0 | 100 | 30 | 15 |
| 16 | 11 | 0 | 0 | 0 | 100 | 80 | 45 |
| 17 | 9 | 0 | 0 | 0 | 70 | 55 | 30 |
| 18 | 1 | 0 | 0 | 0 | 85 | 55 | 30 |
| 19 | 12 | 0 | 0 | 0 | 70 | 30 | 15 |
| 20 | 18 | 0 | 0 | 0 | 100 | 30 | 45 |
aNo randomized
bRandomized.
The central value (zero level) chosen for experiment design were solvent concentration 85% (v/v), extraction temperature 55 °C and extraction time 30 min. in developing the regression equation, the test factors were coded according to the equation.
 |
i |
Where Xi is the coded value of the ith independent variable, 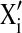 is the natural value of the ith independent variable,
is the natural value of the ith independent variable, 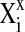 is the natural value of the ith independent variable at the center point and ΔXi is the step change value or difference between maximum and minimum value.
is the natural value of the ith independent variable at the center point and ΔXi is the step change value or difference between maximum and minimum value.
The following quadratic response function for n variables with interaction terms was considered for the mathematical relationship between independent & dependent variables.
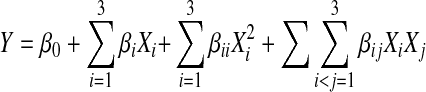 |
ii |
Where Y is the measured response, β0, βi, βii, and βij are the regression coefficients for intercept, linear, quadratic and interaction terms, respectively and Xi and Xj , where Xi and Xj are the coded value of the ith & jth independent variables. The variable XiXj represents the first order interaction between Xi and Xj for (i<j).
Selection of relevant variables and experimental ranges
The initial step was selecting the relevant factors in antioxidants extraction and the experimental ranges for the independent variables. The selection of the solvent type was crucial because different studied showed that there was potential effects of the extracting solvent on an antioxidant activity estimation for the wheat bran. Most of researchers studied different nutraceutical present in different fractions of the wheat by using ethanol, methanol, acetone and water as a solvent. Liyana-Pathirana and Shahidi (2005) already explored the optimization of extraction of the phenolic compounds present in the wheat by using ethanol/water. Absolute ethanol and 50%acetone have been used to prepare antioxidant extracts from wheat and wheat-based cereal products (Yu et al. 2002a, b), while 70% methanol and 50% acetone are widely accepted solvents for extracting phenolic compounds. Therefore, the extraction of antioxidants was carried out by using methanol as an extraction solvent from the Indian hard wheat variety. The experimental ranges for the independent variables were selected as concentration of the solvent in range of 70–90% v/v, temperature in range of 30–80 °C, time in range of 15–45 min with respect to the reported literature (Yu 2007).
The CCRD was chosen to design a series of experiments to provide data to determine the relationship between the responses (i.e free phenolic content, total flavonoid content, total antioxidant activity, ferric reducing power and hydrogen peroxide scavenging activity) and the three process parameters, with the specific ranges (Table1).
The coded and corresponding actual values were used to determine the actual levels of the variables for each of the 20 experiments as given in Table 1.
Extraction of total antioxidants
Bran from WH 711 was obtained by milling the grain in local conventional grinding mill, followed by sieving (60 mesh) to separate flour from bran. 10 gm of wheat bran sample (WH 711) was dissolved in 200 ml of the solvent and then heat it for required time-temperature combination as per the RSM experimental conditions as shown in Table 1. Each time, the mixture was filtered through Whatman filter paper (640 d, slow) and the supernatant was concentrated using a rotary evaporator at 40 °C and then the final volume was adjusted to 40 ml. The antioxidants extract were kept in the dark under refrigerated condition until further analysis.
Analyses of the response variables
Free phenolic compound (FPC)
FPC of the wheat bran extracts was determined following the method of Singleton and Rossi (1965). Briefly, reaction mixture contained 200 μl bran extracts, 800 μl freshly prepared diluted (1:10) Folin–ciocalteu reagent, and 2 ml of 20% sodium carbonate. Volume of the resulting mixture was adjusted to 10 ml and placed in dark for 2 h to ensure completion of reaction. The absorbance of resulting blue-colored mixture was measured at 760 nm against sodium carbonate (20%) as a blank by using a spectrophotometer (UV5704SS, ECI, India), with 1 cm cell. Gallic acid was used as calibration standard and results were calculated as gallic acid equivalents (mg/g) of bran.
Total flavonoids content (TFC)
TFC of the wheat bran extracts was determined following the method of Nieva Moreno et al. (2000). Aliquots of diluted extracts (0.5 ml) were added to test tubes and mixed with 0.1 ml of 10% aluminium nitrate, 0.1 ml of 1 M aqueous potassium acetate and 4.3 ml of 80% methanol. The reaction mixture was kept for 40 min at room temperature and then the absorbance of the reaction mixtures was measured at 415 nm. 80% methanol was used as blank. Catechin was used as a standard compound to construct a standard curve and results were calculated as catechin equivalent (mg/g) of bran.
Total antioxidant activity (TAA)
TAA of the wheat bran extracts was evaluated by the phosphomolybdenum method (Jayaprakasha et al. 2002). The assay is based on the reduction of Mo (VI) – Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acidic pH. The extract 0.3 ml was combined with 3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95 °C for 90 min. Then the absorbance of the solution was measured at 695 nm using spectrophotometer (UV5704SS, ECI, India) against blank after cooling to room temperature. Methanol (0.3 ml) in the place of extract was used as the blank. The antioxidant activity is expressed as mmol ascorbic acid equivalents per gram of bran.
Ferric reducing power (FRP)
The FRP of wheat bran extracts was determined following the method of Oyaizu (1986) with some modifications. The assay medium contained 1 mL of wheat bran extract in 2.5 ml of the 0.2 M phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide. After incubation at 50 °C for 20 min, 2.5 mL of 10% trichloroacetic acid were added to the mixture followed by centrifugation for 10 min. One millilitre of the supernatant was mixed with 2.5 mL distilled water and 0.5 mL of 0.1% ferric chloride, and then the absorbance of the resultant solution were noted at 700 nm against phosphate buffer (6.6) as blank. A standard curve was prepared using various concentrations of ascorbic acid and the reducing power was expressed as mmol ascorbic acid equivalents per gram of bran.
Hydrogen peroxide scavenging activity (HPSA)
The HPSA assay was carried out following the procedure of Ruch et al. (1989). Wheat bran extract (2.5 ml) were dissolved in 3.4 mL of 0.1 M phosphate buffer (pH 7.4) and mixed with 0.6 mL of a 43 mM solution of hydrogen peroxide prepared in the same buffer. The absorbance of the reaction mixture was recorded over a 40-min period at 10 min intervals at 234 nm. A blank sample devoid of hydrogen peroxide was used for background subtraction. The concentration of hydrogen peroxide in the assay medium was determined using a standard curve of tocopherol and antioxidant activity was expressed as mmol hydrogen peroxide scavenged per gram of bran.
Optimization and validation of the model
‘Design expert—7.1.6’ software was used for regression and graphical analysis of the data obtained. The optimum values of the selected variables were obtained by solving the regression equation and also by analysing the response surface contour plots. The verification of the validity and adequacy of the predictive extraction model was realized in these optimum conditions of solvent composition, temperature and time of contact.
Results and discussion
Fitting the models
In RSM, natural variables are transformed into coded variables that have been defined as dimensionless with a mean zero and the same standard deviation (Liyana-Pathirana and Shahidi 2005).The experimental and predicted values for responses (FPC, TFC, TAA, FRP, and HPSA) under different combination of extraction conditions are given in Table 2. The results showed that FPC, TFC, TAA, FRP and HPSA of hard wheat bran ranged from 0.30 to 1.04 mg (gallic acid equivalent- GAE)/g bran, 0.072–0.510 mg Catechin equivalent per g bran, 0.0082–0.016 mM ascorbic acid equivalent/g bran, 0.77–2.67 mM ascorbic acid equivalent /g bran and 0.32–3.17 mM tocopherol equivalent /g bran respectively for the samples treated under different extraction conditions as shown in Table 2.
Table 2.
Experimental and predicted values for the responses FPC, TFC, TAA, FRP and HPSA under different extraction conditions
| Std.a order | FPCb | TFCc | TAAd | FRPe | HPSAf | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp.g | Pred.h | Exp.g | Pred.h | Exp.g | Pred.h | Exp.g | Pred.h | Exp.g | Pred.h | |
| 1 | 0.30 | 0.27 | 0.072 | 0.059 | 0.0094 | 0.0092 | 0.77 | 0.77 | 0.32 | 0.15 |
| 2 | 0.36 | 0.34 | 0.12 | 0.10 | 0.0082 | 0.0078 | 1.35 | 1.23 | 0.75 | 0.78 |
| 3 | 0.45 | 0.47 | 0.15 | 0.15 | 0.012 | 0.012 | 1.12 | 1.06 | 0.67 | 0.77 |
| 4 | 0.50 | 0.51 | 0.22 | 0.23 | 0.011 | 0.011 | 1.57 | 1.62 | 1.46 | 1.41 |
| 5 | 0.37 | 0.36 | 0.11 | 0.10 | 0.011 | 0.011 | 0.96 | 0.90 | 0.47 | 0.49 |
| 6 | 0.46 | 0.44 | 0.15 | 0.15 | 0.0097 | 0.0097 | 1.32 | 1.37 | 1.09 | 0.97 |
| 7 | 0.56 | 0.58 | 0.18 | 0.20 | 0.010 | 0.011 | 1.08 | 1.19 | 1.20 | 1.15 |
| 8 | 0.59 | 0.63 | 0.26 | 0.27 | 0.010 | 0.010 | 1.77 | 1.75 | 1.48 | 1.63 |
| 9 | 0.43 | 0.42 | 0.11 | 0.11 | 0.013 | 0.013 | 0.80 | 0.80 | 0.58 | 0.68 |
| 10 | 0.49 | 0.48 | 0.18 | 0.17 | 0.012 | 0.012 | 1.27 | 1.31 | 1.26 | 1.24 |
| 11 | 0.69 | 0.76 | 0.32 | 0.36 | 0.011 | 0.012 | 2.12 | 2.25 | 2.13 | 2.37 |
| 12 | 1.04 | 0.96 | 0.51 | 0.47 | 0.014 | 0.013 | 2.67 | 2.58 | 3.17 | 3.01 |
| 13 | 0.79 | 0.81 | 0.35 | 0.37 | 0.014 | 0.014 | 2.14 | 2.27 | 2.59 | 2.69 |
| 14 | 0.94 | 0.92 | 0.44 | 0.41 | 0.016 | 0.015 | 2.49 | 2.40 | 2.99 | 2.97 |
| 15 | 0.82 | 0.86 | 0.38 | 0.40 | 0.014 | 0.015 | 2.25 | 2.28 | 2.68 | 2.78 |
| 16 | 0.84 | 0.86 | 0.39 | 0.40 | 0.015 | 0.015 | 2.38 | 2.28 | 2.90 | 2.78 |
| 17 | 0.89 | 0.86 | 0.42 | 0.40 | 0.015 | 0.015 | 2.24 | 2.28 | 2.75 | 2.78 |
| 18 | 0.84 | 0.86 | 0.39 | 0.40 | 0.015 | 0.015 | 2.27 | 2.28 | 2.77 | 2.78 |
| 19 | 0.87 | 0.86 | 0.38 | 0.40 | 0.015 | 0.015 | 2.27 | 2.28 | 2.84 | 2.78 |
| 20 | 0.89 | 0.86 | 0.42 | 0.40 | 0.015 | 0.015 | 2.38 | 2.28 | 2.91 | 2.78 |
aStandard run - non randomized
bFree phenolic content in mg GAE/g B
cTotal Flavonoid content in mg CE/g B
dTotal antioxidant activity in mM AAE/g B
eFerric reducing power in mM AAE/g B
fHydrogen peroxide activity in mM TE/g B
gExperimental value
hPredicted value
Data expressed as avearge of triplicate meaurements
The maximum FPC, TFC, FRP and HPSA were obtained when the solvent concentration, extraction temperature and extraction time were 85%v/v, 80 °C, 30 min while minimum was at 70%v/v, 30 °C, 15 min (Table 2). TAA was maximum when the solvent concentration, extraction temperature and extraction time were 85%v/v, 55 °C, 45 min while minimum was at 100%v/v, 30 °C, 15 min (Table 2).
The parameters of regression equations obtained by fitting of FPC, TFC, TAA, FRP and HPSA data are given in Table 3. The fitness and adequacy of the model was judged by the coefficient of determination (R2) and the significance of lack-of-fit. R2 which can be defined as the ratio of the explained variation to the total variation was a measure of the degree of fit (Chan et al. 2009). The closer the R2 value to unity, the better the empirical model fits the actual data. The coefficient of determination, R2, were 0.977, 0.980, 0.962, 0.985 and 0.988 for the regressed models predicting the FPC, TFC, TAA, FRP and HPSA respectively, suggesting a good fit. The predicted models seemed to reasonably represent the observed values. Thus, the responses were sufficiently explained by the models. The absence of lack of fit for all responses also strengthened the reliability of the models.
Table 3.
Regression coefficients of predicted quadratic polynomial models for the responses FPC, TFC, TAA, FRP, and HPSA
| Coefficient | FPC | TFC | TAA | FRP | HPSA |
|---|---|---|---|---|---|
| Intercept | 0.86a | 0.40a | 0.015a | 2.28a | 2.78a |
| Linear | |||||
| A | 0.029c | 0.029 b | −4.030E-004 c | 0.26 a | 0.28 a |
| B | 0.097a | 0.055 a | 7.960E-004 b | 0.17 a | 0.32 a |
| C | 0.052b | 0.022 b | 2.490E-004 | 0.067 c | 0.14 b |
| Quadratic | |||||
| A2 | −0.41a | −0.25 a | −2.406E-003 | −1.23 | −1.82 |
| B2 | −3.568E-003 | 0.017 | −2.081E-003 | 0.13 | −0.092 |
| C2 | 1.682E-003 | −3.514E-003 | −4.591E-005 | 0.050 | 0.049 |
| Crossproduct | |||||
| A*B | −8.375E-003 | 6.700E-003 | 1.913E-004 | 0.026 | 1.762E-003 |
| A*C | 2.875E-003 | 1.000E-004 | 7.875E-005 | 7.500E-004 | −0.039 |
| B*C | 4.375E-003 | 1.000E-003 | −6.062E-004 b | −2.500E-004 | 8.413E-003 |
| R2 d | 0.977 | 0.980 | 0.962 | 0.985 | 0.988 |
| Adj. R2 e | 0.957 | 0.963 | 0.929 | 0.972 | 0.977 |
| CVf | 7.19 | 9.46 | 4.87 | 6.01 | 8.18 |
Statistically significant at ap < 0.001, bp < 0.05, and cp < 0.10
dCoefficient of multiple determination
eAdjusted R2
fCoefficient of variance.
The adjusted R2 was a corrected value for R2 after elimination of the unnecessary model terms. If there were many non-significant terms have been included in the model, the adjusted R2 would be remarkably smaller than the R2 (Chan et al. 2009). In this study, the adjusted R2 was for all responses very close to their corresponding R2 value. High values of adjusted R2 also advocated significance of the model for all responses. The coefficient of variation (CV) describes the extent to which the data are dispersed (Liyana-Pathirana and Shahidi 2005). The coefficient of variation is a measure of residual variation of the data relative to the size of the mean; the small values of CV give better reproducibility. The small CV (Table 3) revealed that the experimental results were precise and reliable.
The significance of each coefficient was determined using the F-test and p-value in Table 4. The corresponding variables would be more significant if the absolute F value becomes greater and the p-value becomes smaller (Wang et al. 2007).The F-value for all responses model were greater than the tabulated F value which indicating the adequecy of the models to predict different responses at different extraction conditions.
Table 4.
Analysis of variance of the second-order polynomial model for the responses FPC, TFC, TAA, FRP and HPSA
| Sum of square | |
|---|---|
| FPC | 0.97* |
| TFC | 0.34* |
| TAA | 9.374E-005* |
| FRAP | 7.34* |
| HPSA | 19.07* |
*Statistically significant at p < 0.0001
Analysis of response surface
Free phenolic content (FPC)
Effect of solvent concentration, temperature and time on FPC
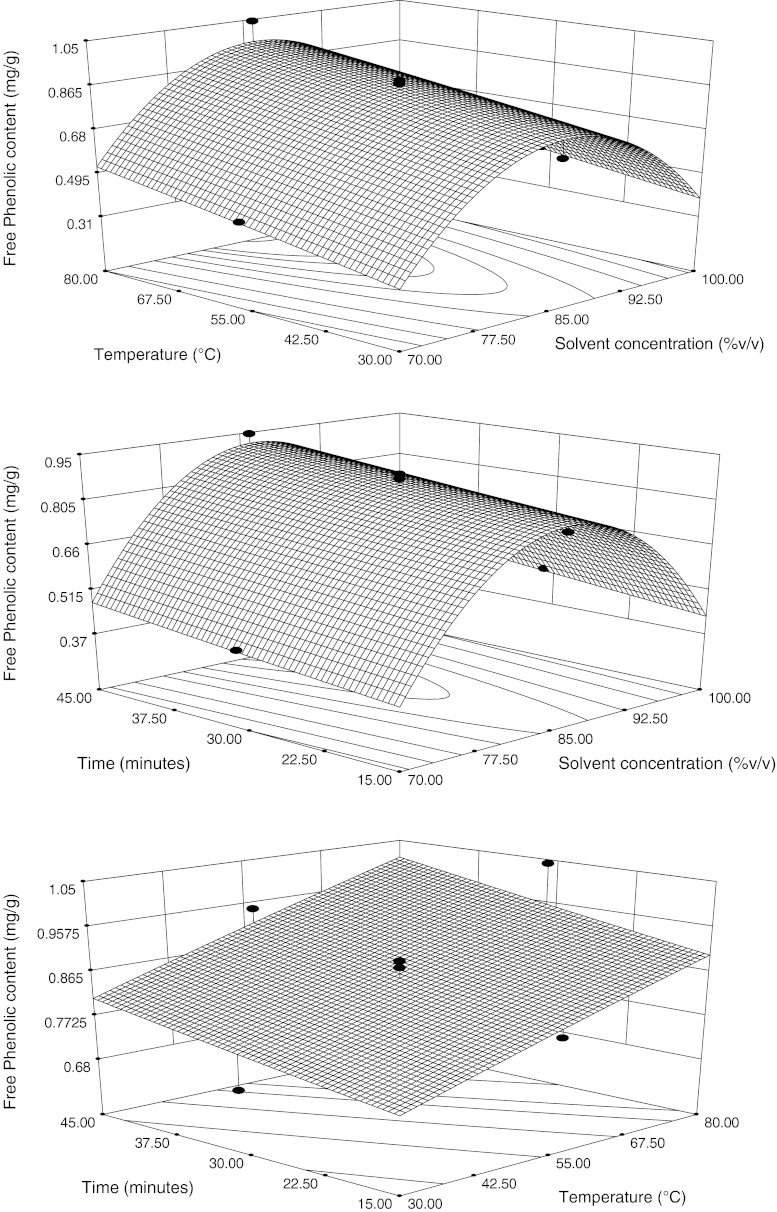
The contour and three-dimensional plots for FPC found in hard winter wheat bran as the response in function of two factors and keeping the other fixed at middle level is shown in Fig. 1. It was evident that the FPC increases with the increased solvent concentration upto 85.7% then decreased at constant time 30 min. It has been established that the extraction yield of phenols is greatly depending on the solvent polarity. Yilmaz and Toledo (2006) found that aqueous solutions of ethanol, methanol or acetone were better than a single compound solvent system for the extraction of the total phenols from Muscadine seed power. Jayaprakasha et al. (2007) reported that ethanol that ranged from 40% to 80% had greater efficiency in the extraction of polyphenol compounds compared to pure ethanol. The results from this study showed that variations in the polyphenol content of various extracts varied widely, depending on the polarities of solvent used and that aqueous solvents were more efficient in extracting FPC, compared to their corresponding absolute solvents, indicating that the wheat bran polyphenols were highly polar compounds. The data indicates that the model can be fitted at quadratic level (p < 0.0001) whereas the model was not significant at linear and interactive level (p > 0.1). Besides, the absence of any lack of fit (p > 0.05) also strengthened the reliability of the models.
Fig. 1.
Response surface and contour plots for Free phenolic content, in function of solvent composition, temperature and time. The value of the missing independent variable in each plot was kept at the centre point
It was observed that FPC increased with the increasing temperature at constant time 30 min (Fig. 1). Increasing temperature may favour extraction by enhancing solubility of phenolic compounds in the solvent. A major effect of the increase of extraction temperature may be too increased in the rate of the extraction thereby decreasing the extraction time (Cacace and Mazza 2002). TPC increased with the increased time (Fig. 1) at constant temperature 55°C. Chan et al. (2009) results showed that an increase in extraction time from 60 to 180 min was accompanied by a small increment in total phenolic content.
Total flavonoid content (TFC)
Effect of the solvent concentration, temperature and time on TFC
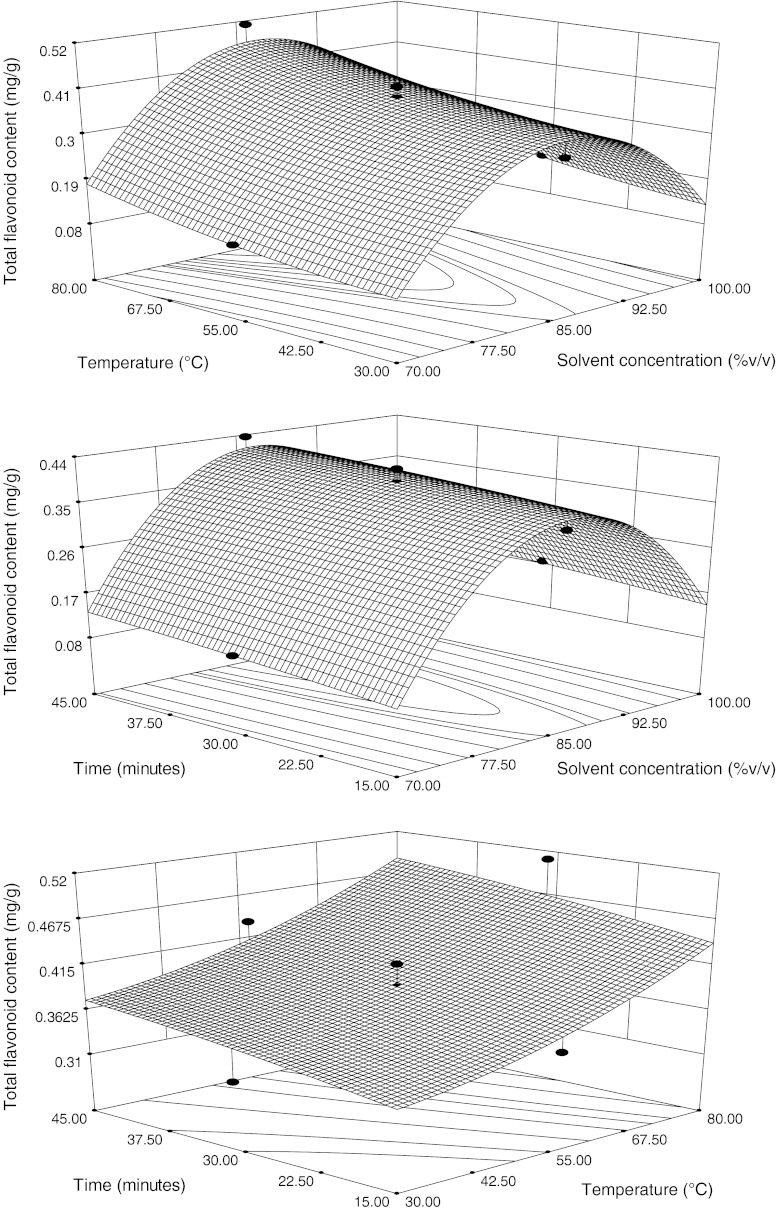
In the model, all independent variables have positive effect on the TFC at linear level (p < 0.005). Effect of the two independent variables on the TFC, when third variable is fixed at middle level, is shown by contour and three dimensional response plots (Fig. 2). It is evident from the figure that the TFC increased with the increased solvent concentration upto 86.13% then decreased at constant time 30 min. According to Kumar et al. (2008) the optimal extraction yield may be fulfilled when the polarity of the fluid and its flavonoids are coincident. They found that TFC increased with the concentration of ethanol 55–75% and beyond 75%, decrease in the flavonoids content was noticed in case of leaves of Tabernaemontana heyneana Wall. Ethanol interacts with the flavonoids probably through non-covalent interactions and promotes a rapid diffusion into the solution (Luque de Castro and Tena 1996). Various concentration of ethanol used exhibited different effect in changing the fluid polarity and thus had diverse effect on the solubility enhancement of the flavonoids (He et al. 2005).
Fig. 2.
Response surface and contour plots for total flavonoid content, in function of solvent composition, temperature and time. The value of the missing independent variable in each plot was kept at the centre point
At fixed time 30 min, TFC increased with the increasing temperature (Fig. 2). Kumar et al. (2008) reported that the contents of flavonoids gradually increased with a rise in the temperature in a range of 55–85 °C with a 10 °C temperature interval. It may be probable that the greater speed of the molecule movements in higher temperature may cause flavonoids diffusion more quickly from cell to extracting agent. He et al. (2005) found that temperature’s effect on extraction is dual. On one hand, higher temperature can accelerate the solvent flow and thus increase the flavonoids content and on the other hand, higher temperature can decrease the fluid density that may reduce the extraction efficiency. The contour and 3D graph (Fig. 2) showed that TFC increased with increasing the time at constant temperature 55°C. Kumar et al. (2008) results showed that the contents of flavonoids extracted for 2 h reached maxima and prolonged extraction may not yield an increased content.
Total antioxidant activity (TAA)
Effect of the solvent concentration, temperature and time on the TAA
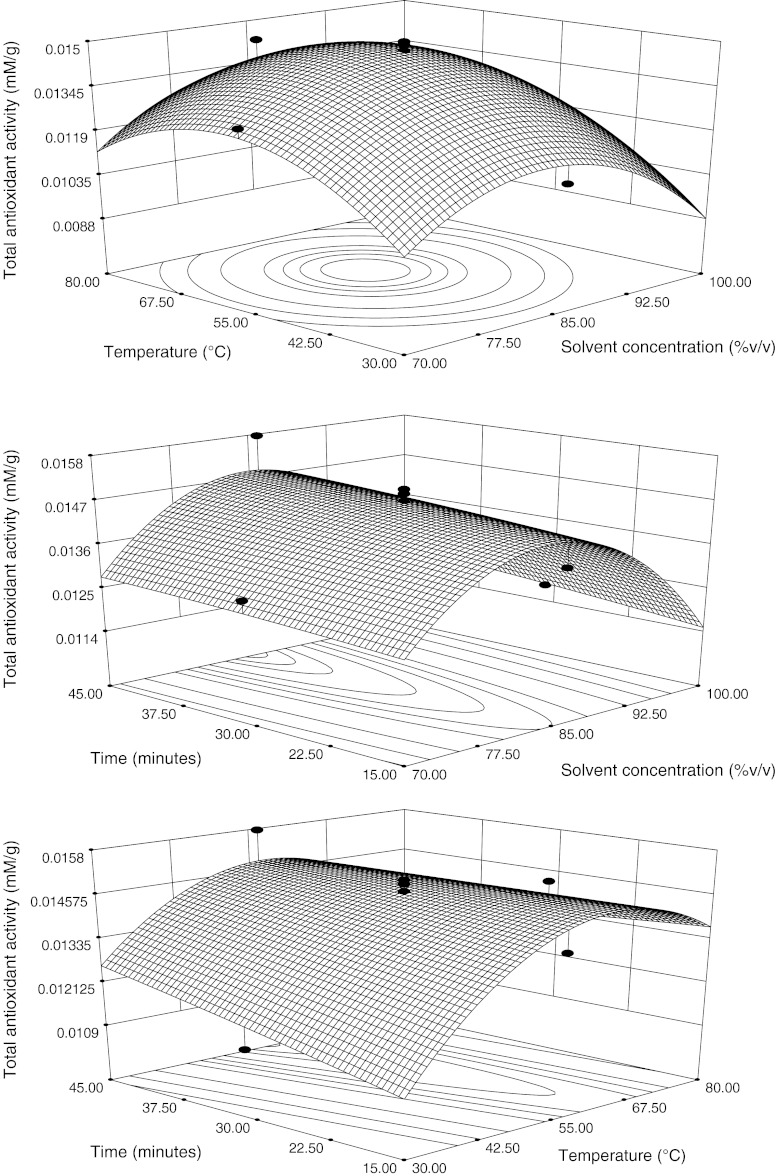
Both solvent concentration and temperature were found to be significantly negative quadratic effect on FPC (p < 0.001). Figure 3 illustrated three-dimensional response surface plots by presenting the response in function of two factors and keeping the other constant at its middle level which showed the effects of the selected parameters on TAA. It is depicted that TAA increased with the increased solvent concentration upto 83.82% then decreased. TAA has been reported to reach a maximum followed by a deceases with further increased in the proportion of the organic solvent in the extraction medium (Liyana-Pathirana and Shahidi 2005).Methanol is usually recommended for the extraction of antioxidant compounds (Iqbal et al. 2005) and its effectiveness could be improved by adding water as co-solvent, particularly, in the protocols, where the extraction of antioxidant compounds of a multifarious nature is mandatory.
Fig. 3.
Response surface and contour plots for total antioxidant activity, in function of solvent composition, temperature and time. The value of the missing independent variable in each plot was kept at the centre point
With regards to the temperature, TAA increased with the increasing temperature upto 60 °C and then begin to decline (Fig. 3). Similar behaviour was also found by Liyana-Pathirana and Shahidi (2005). Results indicated that mobilization of active compounds from the substrate may occur upto certain level followed by their possible loss due to decomposition at higher temperatures. According to Wettasinghe & Shahidi (1999), high temperatures may mobilize certain antioxidants while promoting possible concurrent decomposition of antioxidants which were already mobilized at lower temperature. It was also stated that the rate of extraction of thermally stable antioxidants at elevated temperatures is higher than the rate of decomposition of less soluble antioxidants. This has been suggested by the relatively high antioxidant activities possessed by extracts prepared at higher temperatures. Increasing temperature may favour extraction by enhancing solubility of phenolic compounds in the solvent. TAA increased when extraction time increased from 15 to 45 min (Fig. 3). Liyana-Pathirana and Shahidi (2005) results showed that TAA increased when extraction time increased from 15 to 60 min. Beyond 70 min, TAA decreased sharply and reached minimum at 105 min, possibly due to the decomposition of active compounds during the prolonged extraction time.
Ferric reducing power (FRP)
Effect of the solvent concentration, temperature and time on the FRP
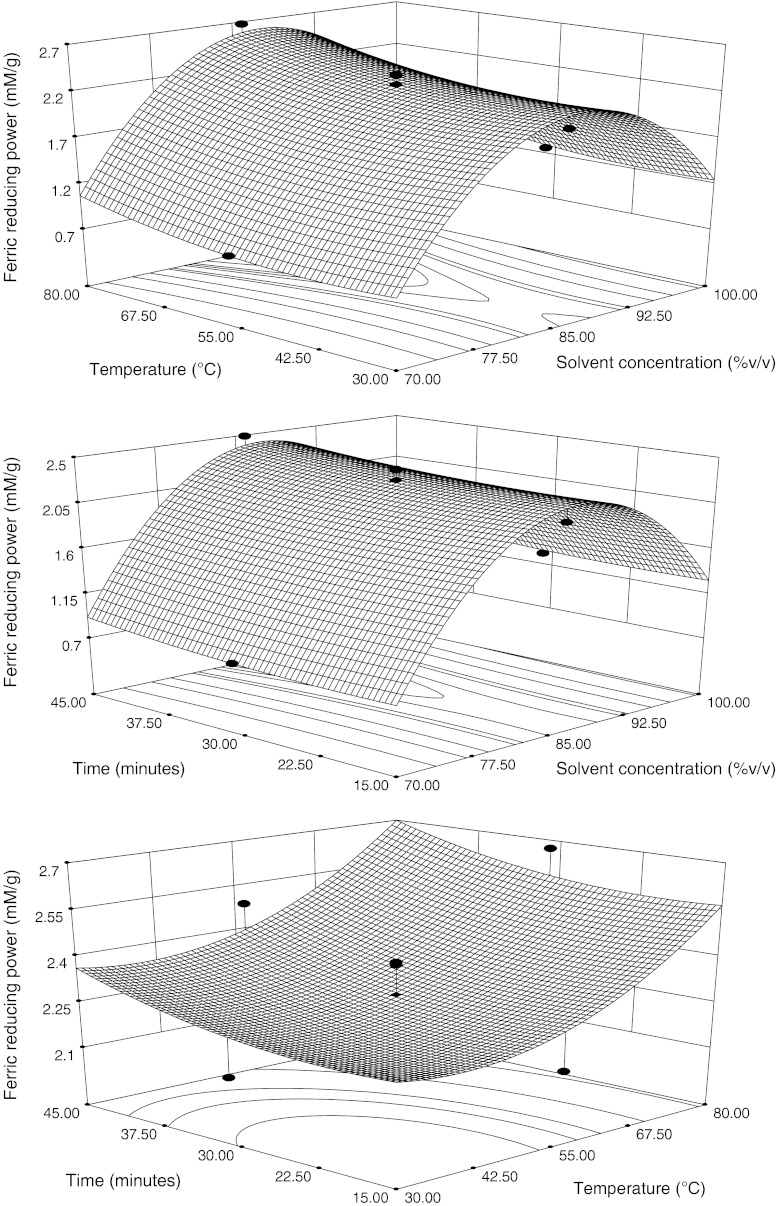
At constant time 30 min, the FRP increased with the increased solvent concentration upto 86.79% then decreased (Fig. 4). This conclusion is in agreement with the results of Negi et al. (2005), who reported that methanol extract of sea buckthorn seed had higher reducing power than extracts using low polarity chloroform. Turkmen et al. (2007) also found that polar aqueous solvents dissolve more polar plant polyphenols with higher reducing power at all different extraction times. The reducing potential of antioxidant components is very much associated with their total phenolic content. The plant extracts with higher levels of total phenolics also exhibit greater reducing power (Cheng et al. 2006). The reducing capacity of a compound may reflect its antioxidant potential. It has been reported that the reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (Shahidi 2000). Duh (1998) stated that reductones are efficient reducing agents and their efficiency is attributed to their hydrogen-donating ability. The results on reducing power demonstrate the electron donor properties of wheat extracts thereby neutralizing free radicals by forming stable products. The outcome of the reducing reaction is to terminate the radical chain reactions that may otherwise be damaging (Yen and Chen 1995). In the model, solvent concentration and temperature were found to be significant model terms (P < 0.05) in linear manner.
Fig. 4.
Response surface and contour plots for ferric reducing power, in function of solvent composition, temperature and time. The value of the missing independent variable in each plot was kept at the centre point
Figure 4 indicates that FRP increased with increasing the temperature and time that followed the FPC compound model behaviour. Satoh et al. (2005) observed that there was a strong correlation between the FRP and TPC of different tea extracts including black tea.
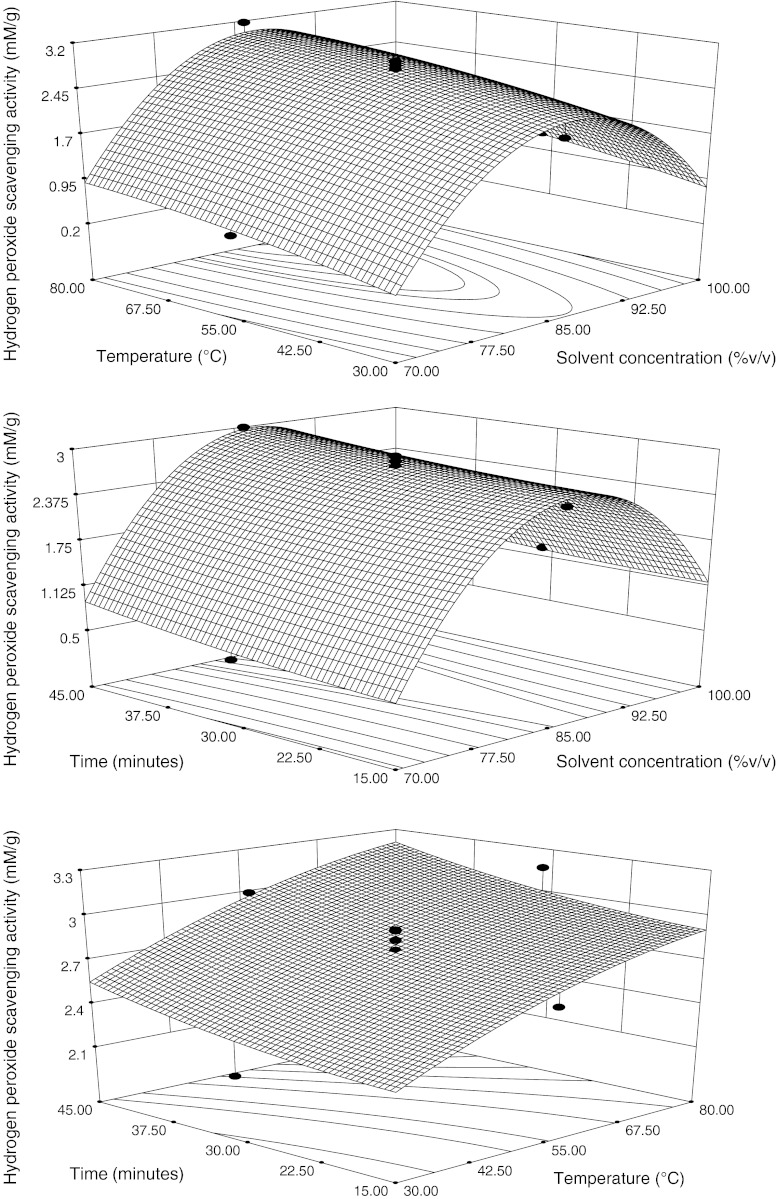
Hydrogen peroxide scavenging activity (HPSA)
Effect of the solvent concentration, temperature and time on the HPSA
Solvent concentration demonstrated a pronounced influence on HPSA in linear and Quadratic manner (p < 0.005) but other two variables (temperature and time) displayed significant effect at linear level (p < 0.005) only. The HPSA increased with the increasing solvent concentration upto 86.18% then decreased at the fixed time 30 min (Fig. 5). Duh et al. (1999) reported results for Chrysanthemum morifolium with high relationship between TPC and HPSA of the water extracts. The extracts were capable of scavenging hydrogen peroxide in a concentration—dependent manner. Hydrogen peroxide itself is not very reactive, but sometimes is toxic to cells because it may give rise to hydroxyl radical in the cells. Dietary polyphenols have also been shown to protect mammalian and bacterial cells from cytotoxicity induced by hydrogen peroxide, especially compounds with the o-dihydroxy phenolic structure such as quercetin, catechin and, gallic acid and caffeic acid ester. Therefore, the flavonoid compounds of V. negundo extract along with carotene and ascorbic acid present therein may probably be involved in removing the H2O2 (Alam et al. 2009). Hydrogen peroxide (H2O2) generates singlet oxygen (1O2) and a hydroxyl radical (•OH), which then become very powerful oxidizing agents. Not only 1O2 and HO· but also H2O2 can cross membranes and may oxidize a number of compounds. While H2O2 itself is not that reactive, it can generate the highly reactive hydroxyl radical through the Fenton reaction. Thus, the scavenging of H2O2 is an important antioxidant defence mechanism. The decomposition of H2O2 to H2O involves the transfer of electrons (Alam et al. 2009).
Fig. 5.
Response surface and contour plots for hydrogen peroxide scavenging activity, in function of solvent composition, temperature and time. The value of the missing independent variable in each plot was kept at the centre point
With regards to the temperature, HPSA increased with increasing the temperature is presented in Fig. 5 that followed the FPC model behaviour. Duh et al. (1999) reported results for Chrysanthemum morifolium with high relationship between TPC and HPSA of the water extracts. The contour and 3D graphs (Fig. 5) shows that HPSA increased with the increasing time. The flavonoid compounds of V. negundo extract along with carotene and ascorbic acid present therein may probably be involved in removing the H2O2 (Alam et al. 2009). Kumar et al. (2008) results showed that the contents of flavonoids extracted for 2 h reached maxima and prolonged extraction may not yield an increased content.
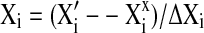
Optimization of parameters to maximize antioxidants extraction
Design expert software 7.1.6 was used to optimize the antioxidants extraction conditions like solvent concentration, temperature, time to maximize extraction of nutraceutical potentials like FPC, TFC, TAA, FRP and HPSA. The software uses second order model to optimize the responses. Predicted values of different responses on optimum conditions (in the range constraint) are given in Table 5. When constaint in the range were selected then the optimum conditions were found as 85.78% v/v solvent concentartion, 74.35 °C temperature and 45 min time with the desirability of 90.4%. But in practice, however, it is difficult to maintain the recommended conditions during processing and some deviation is expected. Therefore, optimum conditions were targeted as 85% v/v solvent concentartion, 75 °C temperature and 45 min time with a desirability of 93.8%. Predicted values of different responses on targeted optimum conditions are given in Table 5. Response surface and contour plots for desirability, in function of solvent composition and temperature and the value of the missing independent variable in plot was kept at the centre point is shown in Fig. 6. In case of targeted value of constraint, it was observed that predicted values of TAA, FRP and HPSA values were marginally lower than in range constraint predicted values whereas targeted constraint had provided little higher predicted values of the FPC, TFC and desirability.
Table 5.
Optimized level (in the range), optimum level (targeted), predicted optimum value and experimental value of FPC, TFC, TAA, FRP, HPSA and desirability
| Optimum value (In the range) | Optimum value (Targeted) | |||
|---|---|---|---|---|
| Variables | Solvent concentration | 85.78% v/v | 85% v/v | |
| Temperature | 74.35 °C | 75 °C | ||
| Time | 45 min | 45 min | ||
| Predicted Value | Experimental value | |||
| Responses | FPC | 0.992 mg/g | 0.994 mg/g | 0.921 mg/g |
| TFC | 0.46858 mg/g | 0.46963 mg/g | 0.4588 mg/g | |
| TAA | 0.013768 mM/g | 0.01370 mM/g | 0.01408 mM/g | |
| FRP | 2.62002 mM/g | 2.61875 mM/g | 2.532 mM/g | |
| HPSA | 3.179 mM/g | 3.1761 mM/g | 3.193 mM/g | |
| Desirability | 0.904 | 0.938 | ||
Fig. 6.
Response surface and contour plots for desirability, in function of solvent composition and temperature. The value of the missing independent variable in plot was kept at the centre point
Verification of predictive model
On the above said optimum condition (target constraint), all experiments were conducted for checking the variation in value of nutraceutical potentials of wheat bran (WH 717). The experimental values of different responses on these optimum conditions of different wheat bran are given in Table 5. which showed that the experimental results were very close to the predicted one. This implied that there was a high fit degree between the values observed in experiment and the value predicted from the regression model. Hence, the response surface modeling could be applied effectively to predict extraction of the nutraceeutical for the hard wheat bran.
Conclusions
Response surface methodology was sucessfully used to determined the optimum antioxidants extraction conditions that yield maximum extraction of different nutraceutical potentials like TPC, TFC, TAA, FRP and HPSA.. The second-order polynomial model gave a satisfactory description of the experimental data. Result showed that aqueous methanol was more efficient in extracting FPC, TFC, TAA, FRP and HPSA as compared to their corresponding absolute one. The optimal conditions for the antioxidants extraction were found to be methanol concentration of 85%v/v, extraction temperature of 75 °C, extraction time of 45 min, for hard wheat bran. Under the optimum conditions, the experimental values of FPC, TFC, TAA, FRP and HPSA were agreed with those predicted, thus indicating suitability of the model employed.
References
- Alam MA, Rahman MM, Subhan N, Majumder MM, Hasan SMR, Kter RM, Majumder EH, Faruque A. Antioxidant potential of the ethanol extract of the leaves of Vitex Negundol L. Turk J Pharm Sci. 2009;6(1):11–20. [Google Scholar]
- Cacace JE, Mazza G. Extraction of anthocyanin and other phenolics from black currants with sulfured water. J Agric Food Chem. 2002;50:5939–5946. doi: 10.1021/jf025614x. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lee CY, Yap CF, Wan Aida WM, Ho CW. Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. Int Food Res J. 2009;16:203–213. [Google Scholar]
- Cheng Z, Su L, Moore J, Zhou K, Luther M, Yin JJ, Yu LL. Effect of postharvest treatment and heat stress on availability of wheat antioxidants. J Agric Food Chem. 2006;54:5623–5629. doi: 10.1021/jf060719b. [DOI] [PubMed] [Google Scholar]
- Duh PD. Antioxidant activity of Budrock (Arctium Laooa Linn): its scavenging effect on free radical and active oxygen. J Am Oil Chem. 1998;75:455–461. doi: 10.1007/s11746-998-0248-8. [DOI] [Google Scholar]
- Duh PD, Tu YY, Yen GC. Antioxidant activity of water extracts of Harng Jyur (Chrisanthemun morifolium Ramat) Lebensm Wiss Technol. 1999;32:269–277. [Google Scholar]
- Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-I. [DOI] [PubMed] [Google Scholar]
- He GQ, Xiong HP, Chen QH, Ruan H, Wang ZY, Traore L. Optimization of conditions for supercritical fluid extraction of flavonoids from hops (Humulus lupulus L.) J Zhejiang Univ Sci. 2005;6B(10):999–1004. doi: 10.1631/jzus.2005.B0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Bhanger MI, Anwar F. Antioxidant properties and components of bran extracts from selected wheat varieties commercially available in Pakistan. Lebensm Wiss Technol. 2005;40:361–367. [Google Scholar]
- Jayaprakasha GK, Negi PS, Jena BS, Rao LJM. Antioxidant and antimutagenic activities of Cinnamomum zeylanicum fruit extracts. J Food Compos Anal. 2007;20:330–336. doi: 10.1016/j.jfca.2006.07.006. [DOI] [Google Scholar]
- Jayprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch. 2002;57c:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- Kim K, Tsao R, Yang R, Cui SW. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effects of hydrolysis conditions. Food Chem. 2006;95:466–473. doi: 10.1016/j.foodchem.2005.01.032. [DOI] [Google Scholar]
- Kumar S, Kumar D, Singh N, Vasisht BD. In vitro, free radicals scavenging and antioxidant activity of Moringa Oleifera pods. J Herb Med Toxicol. 2007;1(2):17–22. [Google Scholar]
- Kumar ST, Baskar R, Shanmugam S, Rajsekaran P, Sadasivam S, Manikandan V. Optimization of flavonoids extraction from the leaves of Tabernaemontana heyneana Wall. using L16 Orthogonal design. Nat Sci. 2008;6(3):14–25. [Google Scholar]
- Li W, Shan F, Sun S, Corke H, Beta T. Free radical scavenging properties and phenolic content of Chinese black-grained wheat. J Agric Food Chem. 2005;53:8533–8536. doi: 10.1021/jf051634y. [DOI] [PubMed] [Google Scholar]
- Liyana-Pathirana CM, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Luque De Castro MD, Tena MT. Strategies for supercritical fluid extraction of polar and ionic compounds. Trends Anal Chem. 1996;15:32–37. doi: 10.1016/0165-9936(96)88035-6. [DOI] [Google Scholar]
- Marama M, Amarowicz R, Weidner S, Abe S, Shahidi F. Antioxidant activity of Triticale caryopses and embryos extracts. Food Sci Biotechnol. 2004;13:421–424. [Google Scholar]
- Mpofu A, Sapirstein HD, Beta T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J Agric Food Chem. 2006;54:1265–1270. doi: 10.1021/jf052683d. [DOI] [PubMed] [Google Scholar]
- Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chem. 2005;92:119–124. doi: 10.1016/j.foodchem.2004.07.009. [DOI] [Google Scholar]
- Nieva Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radicalscavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000;71:109–114. doi: 10.1016/S0378-8741(99)00189-0. [DOI] [PubMed] [Google Scholar]
- Onyeneho SN, Hettiarachchy NS. Antioxidant activity of durum wheat bran. J Agric Food Chem. 1992;40(9):1496–1500. doi: 10.1021/jf00021a005. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Ozddemir M, Ozen BF, Dock LL, Floros JD. Optimization of osmostic dehydration of diced green peppers by response surface methodology. Lebensm Wiss Technol. 2008;41:2044–2050. [Google Scholar]
- Pinzino C. Aging, free radicals, and antioxidants in wheat seeds. J Agric Food Chem. 1999;47:1333–1339. doi: 10.1021/jf980876d. [DOI] [PubMed] [Google Scholar]
- Qu H, Madl RL, Takemoto DJ, Baybutt RC, Wang W. Lignans are involved in the antitumor activity of wheat bran in colon cancer SW480 cells. J Nutr. 2005;135:598–602. doi: 10.1093/jn/135.3.598. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy PK, Bongo A. Antioxidant activity total phenolic and flavonoid content of Morinda Citrifolia fruit extracts from various extraction processes. J Eng Sci Technol. 2007;2(1):70–80. [Google Scholar]
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Satoh E, Tohyama N, Nishimura M. Comparison of the antioxidant activity of roasted tea with green, oolong, and black teas. Int J Food Sci Nutr. 2005;56:551–559. doi: 10.1080/09637480500398835. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolic from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Turkmen N, Velioglu Y, Sedat SF, Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–496. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Chen F, Wu J, Wang Z, Liao X, Hu X. Optimization of pectin extraction assisted by microwave from apple pomace using response surface methodology. J Food Eng. 2007;78:693–700. doi: 10.1016/j.jfoodeng.2005.11.008. [DOI] [Google Scholar]
- Wettasinghe M, Shahidi F. Evening primrose meal: a source of natural antioxidants and scavenger of hydrogen peroxide and oxygen-derived free radicals. J Agric Food Chem. 1999;47:1801–1812. doi: 10.1021/jf9810416. [DOI] [PubMed] [Google Scholar]
- Yen WJ, Chen BH. Isolation of xanthophylls from Taiwanese orange peels and their effects on the stability of soybean oil. Food Chem. 1995;53:417–426. doi: 10.1016/0308-8146(95)99837-P. [DOI] [Google Scholar]
- Yilmaz Y, Toledo RT. Oxygen radical absorbance capacities of grape/wine industry byproducts and effect of solvent type on extraction of grape seed polyphenols. J Food Compos Anal. 2006;19:41–48. doi: 10.1016/j.jfca.2004.10.009. [DOI] [Google Scholar]
- Yu L. Wheat antioxidants. New Jersey: Wiley; 2007. pp. 102–104. [Google Scholar]
- Yu L, Haley S, Perret J, Harris M. Antioxidant properties of hard winter wheat extracts. Food Chem. 2002;78:457–461. doi: 10.1016/S0308-8146(02)00156-5. [DOI] [Google Scholar]
- Yu L, Haley S, Perret J, Harris M, Wilson J, Qian M. Free radical scavenging properties of wheat extracts. J Agric Food Chem. 2002;50:1619–1624. doi: 10.1021/jf010964p. [DOI] [PubMed] [Google Scholar]
- Zhou K, Su L, Yu L. Phytochemical and antioxidant properties in wheat bran. J Agric Food Chem. 2004;52:6108–6114. doi: 10.1021/jf049214g. [DOI] [PubMed] [Google Scholar]
- Zielinski H, Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]