Abstract
Objective
To evaluate the wound healing potential of fractions from ethanol extract of Martynia annua (M. annua) Linn leaves.
Methods
Ethanol extract of M. annua Linn leaves was fractionate into three different fractions (MAF-A, MAF-B and MAF-C) which were screened for wound healing potential using two models: excision and incision on rats. The thin layer chromatography (TLC) profile of all fractions were analyzed and TLC of luteolin was also done. The Povidone-Iodine Ointment was used as reference for comparision. Excision and incision wounds were created on dorsal portion of rats for study. Wound contraction, biochemical parameters (protein level and hydroxyproline level) and histopathological study were performed in excision wound model whereas incision model was used for determination of tensile strength.
Results
The wound contraction and tensile strength of skin tissues were observed significantly greater in MAF-C fraction treated group than other two fractions (P<0.01). In excision wound method (on day 18) protein content and hydroxyproline were found significantly higher in MAF-C group than control group (P<0.01). Histopathological study also showed better angiogenesis, matured collagen fibres and fibroblast cells as compared with the control group.
Conclusions
In conclusion, our findings suggest that fraction MAF-C from ethanol extract of M. annua leaves is found most effective in wound healing.
Keywords: Martynia annua, Wound healing, Povidone-iodine ointment, Incision, Excision, Luteolin, TLC, Wound contraction, Tensile strength
1. Introduction
Looking back over historical aspect of wound healing, we can appreciate that many strides have been made in understanding and treatment especially of acute wounds. Bleeding can be controlled and infection is understood and controlled or overcome. Although we are still unable to sufficiently control and improve the natural healing of acute wounds, we are able to speed epithelization demonstrating that natural healing can be improved and making improved healing a goal of wound healers. Wound healing is a dynamic process that includes three overlapping phases: inflammation, tissue formation and tissue remodeling. It involves soluble mediators, blood cells, extracellular matrix, and parenchymal cells[1]. As the blood components spill into the site of injury, the platelets come into contact with exposed collagen and other elements. This contact triggers the platelets to release clotting factors and essential growth factors. Following hemostasis, the neutrophils enter the wound site and begin phagocytosis to remove foreign materials, bacteria and damaged tissue. As part of this inflammatory phase, the macrophages appear and continue the process of phagocytosis. Once the wound site is cleaned out, fibroblasts migrate in to begin the tissue formation and deposit new extracellular matrix. The new collagen matrix then becomes cross-linked and organized during the final remodeling phase[2].
Though a large number of pharmacological preparations are used to treat serious complications such as infection or to reduce the inflammation, they do not directly cause repair of damaged tissues[3].
The plant Martynia annua (M. annua) Linn. (Family: Martyniaccae), a herbaceous erect, branched, glandular hairy annual herb. Fruits are hard, woody with 2-sharp re-curved hooks and seeds are oblong[4]. It is commonly known as Bichchhu, used in epilepsy and applied locally to tuberculosis glands of camel's neck. The juice of leaves is used as a gargle for sore throat, fruit in inflammation, leaf paste has beneficial effect when applied to the bites of venomous insects and wounds of domestic animal[5]. The root extract exhibits fungicide activity against Alternaria alternata and Aspergillus niger. Chemical examination of M. annua plant reveals the presence of glycosides, tannins, carbohydrates, phenols, flavonoids and anthocyanins. Flowers contain cyanidin-3-galactoside whilst p-hydroxy benzoic acid, snapic acid, and gentisic acid are present in leaves and fruits, respectively[6]. The leaves contain chlorogenic acid and fatty acids (palmitic acid, stearic acid and arachidic acid) are present in seeds. The anti-inflammatory activity of ethanol extract of Martynia diandra Glox was reported in both acute and sub-acute inflammatory process[7]. The present study was aimed for wound healing potential of fractions from ethanol extract of M. annua Linn Leaves.
2. Materials and methods
2.1. Plant material
The leaves of M. annua Linn. were collected adjoining the campus of University, during September to October. The plant was identified and a voucher specimen (No. Bot/Her/267) was deposited in Department of Botany, Dr. H. S. Gour University Sagar, M. P. (INDIA). The plant material was dried in shade, powdered and sieved through 40-mesh size and stored in well-closed container.
2.2. Extraction and fractionation
The powdered plant materials of M. annua L. leaves (2 kg) were extracted in a soxhlet apparatus with petroleum ether [(60-80) °C] for defatting. The defatted dried powdered material was extracted with ethanol (95%) for 3-4 days. The filtrate ethanol extract was concentrated and completely dried (7.1% w/w). Qualitative analysis of ethanol extract was carried out to find out the presence of various phytoconstituents. The dried ethanol extract was treated with chloroform repeatedly to obtain chloroform soluble fraction (fraction A) and chloroform insoluble fraction. The chloroform insoluble fraction was dissolved in methanol and filtered repeatedly to obtain methanol insoluble (fraction B) and methanol soluble fraction (fraction C). All fractions A, B and C were concentrated up to semisolid and subjected for pharmacological screening of wound healing activity.
2.3. Chromatographic study
The chromatographic study was carried out to obtain the number of constituents present in fractions. The TLC of luteolin was done in all fractions. The thin layer chromatography (TLC) of M. anuua extract fractions (MAF-A, MAF-B, MAF-C) were carried out on a pre-coated silica gel plate (0.2 mm, Merck 60 F 254, Germany) as the stationary phase and toluene: chloroform: acetone (40:25:35) as the mobile phase. The extract fractions and standard compound luteolin (LGC Promochem India Private LTD, Bangalore) were spotted on TLC plate and plates were observed in the UV 254 nm.
2.4. Animals and experimental protocol
Wistar albino rats [(150-200) g] of either sex were selected for experiment. They were housed individually in well-ventilated, temperature controlled [(26±2) °C] animal room for seven days prior experiment. The animals were given the standard commercial pellet rodent diet (Hindustan Lever Pvt Limited, Bangalore, India) and water ad libitum. The procedures were reviewed and approved by the Institutional Animal Ethics Committee (Reg. NO. 379/01/ab/CPCSEA).
For excision and incision wound models, 30 animals were randomly selected for each model and divided into 5 groups, with 6 rats in each group. Animals in group 1 were without treatment and served as control. Animals in group 2 received application of M. annua fraction A (MAF-A), animals of group 3 received application of M. annua fraction B (MAF-B), and animals in group 4 received application of M. annua fraction C (MAF-C). The group 5 received 5% w/w Povidone Iodine Ointment USP (Zenith Drugs Pvt Ltd, India) and served as reference group. The extract fractions and reference ointment were applied twice daily to treat different groups of animals. Healing property was assessed using physical, biochemical parameters along with histopathological study. In excision wound, percent wound contraction was determined in every two days interval the tensile strength was determined in incision wound.
2.5. Excision wound model
All animals were anaesthetized by open mask method with anesthetic ether before wound creation. An excision wound was inflicted by cutting away a 500 mm2 full thickness of skin from a predetermined area. The wound was left undressed to the open environment. In this model wound contraction and wound closure time were monitored. Wound contraction was measured as percent contraction in every two days after wound formation. From the healed wound, a specimen sample of tissue was cutout from each rat and stored in 10% formalin for the biochemical parameters (hydroxyproline and protein content) and histopathological study[8].
2.6. Incision wound model
This model was selected to determine the breaking strength of incision wound. All animals were anaesthetized before wound creation and on dorsal portion long incision wound was made through the skin. No local or systemic antimicrobials were used throughout the experiment. Both edges of wound were kept together and stitched with black silk surgical thread (No. 000) and a curved needle (No. 11) was used for stitching. The continuous threads on both wound edges were tighten for good closure of the wound. After stitching, wound was left undressed and all groups were treated as the same as in excision method up to wounds were healed completely. After complete healing sutures were removed on day 9 and tensile strength of healed wound skin was measured using Tensiometer[9],[10].
2.7. Percent wound contraction and epithelialization time
After wound creation the wound margin were traced at 2 days intervals on transparent graph paper having a millimeter scale that was measured by a caliber with an accuracy of 1/20 mm and measurements were continued up to complete healing. After two days healed area was calculated by subtracting initial wound area to the unhealed area[11]. Contraction was represented as percent wound contraction and epithelialization time was observed after complete healing.
2.8. Tensile strength
The tensile strength of wound represents the degree of wound healing. An instrument, Tensiometer was used for the measurement, on the basis of Kuwano' method[12]. The extract fractions along with the reference were applied daily for 9 days and tensile strength of treated incision wound was determined and compared with control and reference group. Tensile strength increment indicates better wound healing stimulated by applied topical application.
2.9. Histopathological study
In excision method wound tissue specimens from control, treated and reference groups were collected and stored in 10% formalin after usual processing. 6 µm thick sections were cut and stained with haematoxylin and eosin[13]. The histopathology criteria used for each animal were epithelial proliferation, granulation tissue formation and organization, newly formed capillaries (identified by the presence of erythrocytes in their lumen) per site. Sections were qualitatively assessed under light microscope and fibroblast proliferation, collagen maturation, angiogenesis and epithelialization were observed.
2.10. Protein estimation
The tissue lysate was treated with a mixture of sodium tartrate, copper sulphate and sodium carbonate. This was left to stand for 10 min and then treated with Folin-Ciocalteau reagent that resulted in a bluish color in 20-30 min. The absorbance was measured in UV (Cintra) Spectrophotometer at 660 nm[14].
2.11. Hydroxyproline measurement
Wound tissues were analyzed for hydroxyproline content which is a basic constituent of collagen. Tissues were dried in a hot air oven at (60-70) °C to constant weight and hydrolysed in 6 N HCl at 130 °C for 4 h in sealed tubes. The hydrolysate was neutralized to pH 7 then subjected to chloramine-T oxidation for 20 min[15]. The reaction was terminated by addition of 0.4 M perchloric acid and developed color with Ehlrich reagent at 60 °C and read at 557 nm in UV (Cintra) Spectrophotometer.
2.12. Statistical analysis
Pharmacological data were expressed as mean±SD for six rats and data were evaluated using the Dunnett test. Values of P<0.01 were considered to be statistically significant.
3. Results
3.1. Chromatographic study
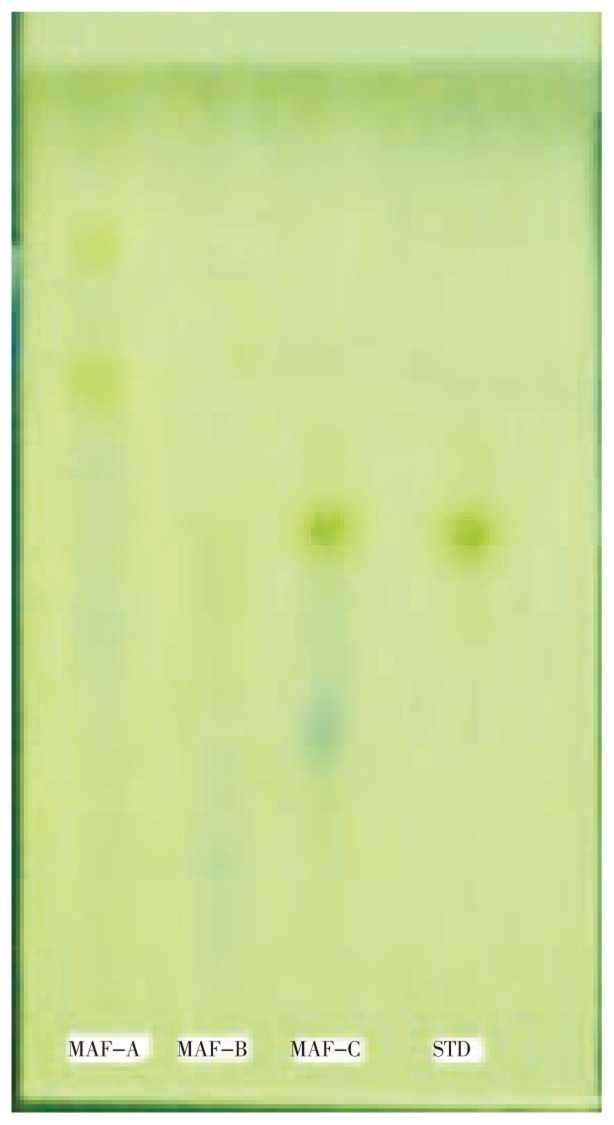
The TLC of three fractions (MAF-A, MAF-B and MAF-C) of M. annua ethanol extract and standard luteolin flavonoid compound was performed and Rf value of luteolin was 5.7. The luteolin was found present in only MAF-C fraction that was confirmed by Rf value and color of spot (Figure 1).
Figure 1. TLC of M. annua Linn ethanol extract fractions (MAF-A, MAF-B and MAF-C) with standard luteolin. STD: luteolin.
3.2. Wound contraction and epithelialization time
The percent wound contraction was determined using the following formula:
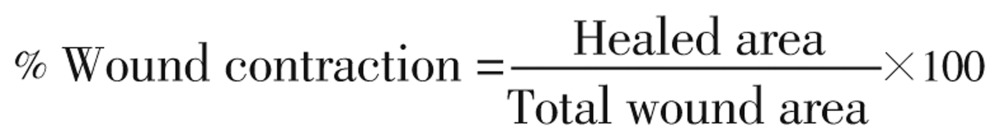 |
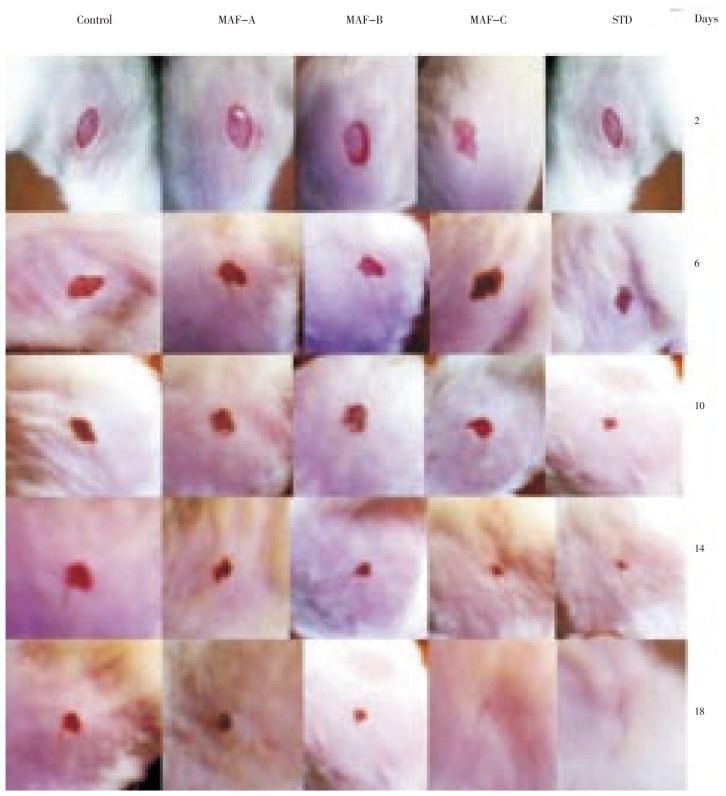
Measurement taken in every two days interval after fractions application continued until wound was completely healed. On day 18 reference and MAF-C treated groups showed 100% wound contraction. The fraction MAF-A, MAF-B treated and control groups were found 92.43%, 97.52% and 84.62% wound contraction, respectively (Table 1). On day 18 no scars were observed in treated groups with MAF-C fraction and reference ointment, which was an indication for complete healing (Figure 2).
Table 1. Effect of different extract fractions of M. annua Linn leaves and reference ointment on % wound contraction and epithelialization period of excision wound in rats (Mean±SD) (n=6).
| Groups | Percent wound contraction |
Epithelialization period | |||||||||
| 2 day | 4 day | 6 day | 8 day | 10 day | 12 day | 14 day | 16 day | 18 day | 20 day | ||
| Control | 7.13 ±0.83 | 15.06±0.56 | 25.61±1.32 | 34.04±0.97 | 41.14±1.57 | 50.22±1.88 | 66.71±0.58 | 78.93±2.13 | 84.62±1.48 | 90.37±2.07 | 24 |
| MAF A | 10.62±0.79 | 28.33±1.62 | 41.52±1.55 | 54.12±1.39 | 69.73±2.44 | 74.45±0.76 | 80.17±1.88 | 88.81±1.07 | 92.43±2.47 | 100.00 | 20 |
| MAF B | 18.41±0.38 | 25.73±0.57 | 36.51±1.22 | 49.05±2.75 | 56.67±1.46 | 72.44±0.76 | 86.34±1.57 | 93.53±0.89 | 97.52±1.58 | 100.00 | 20 |
| MAF C | 10.75±1.52 | 26.50±1.58 | 35.49±2.41 | 51.87±0.87 | 67.48±1.74 | 79.68±1.88 | 82.14±0.98 | 90.31±2.32 | 100.00 | - | 18 |
| Reference ointment | 11.37±1.30 | 25.65±0.87 | 40.82±1.05 | 56.44±1.54 | 67.11±0.86 | 78.59±1.76 | 84.42±1.72 | 97.75±1.72 | 100.00 | - | 18 |
P<0.01 as compared with control group.
Figure 2. Wound areas of different groups in excision wound method.
STD: Povidone Iodine Ointment.
3.3. Tensile strength
The tensile strength of incision wound tissues in all fractions and reference ointment treated groups was measured on day 9 and significantly increased in all fractions and reference groups than the control group (P<0.01) (Table 2).
Table 2. Effect of different extract fractions of M. annua Linn leaves and reference ointment on various wound parameters of incision and excision wound in rats (Mean±SD) (n=6).
| Groups | Hydroxyproline content (mg/g tissue) | Protein content (mg/g tissue) | Tensile strength (gm/cm2) |
| Control | 21.74±1.85 | 47.30±1.72 | 423.00±10.96 |
| MAF A | 37.11±1.25 | 56.30±0.55 | 603.00±12.01 |
| MAF B | 32.86±0.85 | 52.50±1.70 | 635.00±9.68 |
| MAF C | 42.01±0.82 | 83.60±0.72 | 850.00±11.89 |
| Reference ointment | 45.27±1.56 | 85.90±1.57 | 973.00±12.08 |
P<0.01 as compared with control group.
3.4. Hydroxyproline measurement
The hydroxyproline content of wound tissue on day 18 was determined. The hydroxyproline content of MAF-C treated and reference groups was significantly higher in comparison with control group as well as MAF-A and MAF-B fraction treated groups (P<0.01) (Table 2).
3.5. Protein estimation
The protein level of MAF-C, MAF-B, MAF-A treated and reference groups were significantly higher in comparison with control group (P<0.01) (Table 2).
3.6. Histopathological study
On day 18 MAF-C treated and reference groups were observed for complete epithelialization. Injury in both MAF-C treated and reference groups was found to be covered by new epidermal layers. The proliferation of collagen, fibrous tissue and capillaries with epidermal covering at the margin of wound were observed. Formation of dense fibrous tissue and blood capillaries, arrangement of capillaries was perpendicular to the fibrous tissue, mature connective tissue and a few capillaries with thick epidermal lining at the periphery of the wound were observed (Figure 3 and 4). A number of fibroblast were clearly observed in the dermis and the process of epidermal regeneration was completed. Edema, monocyte cells and cellular necrosis were observed in the control group (Figure 5). In MAF-A, MAF-B and control groups, moderate number of fibroblast were observed in the dermis and less new blood vessels formations were observed (Figure 5, 6 and 7).
Figure 3. Photomicrograph of wound tissue with MAF-C fraction treatment in excision method.
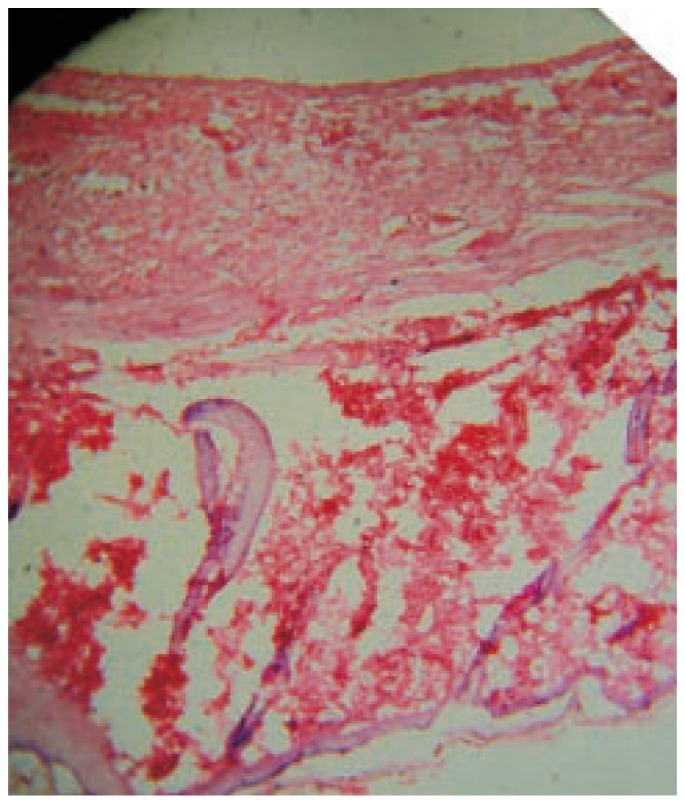
Hematoxylin and eosin stain (100×).
Figure 4. Photomicrograph of wound tissue with reference ointment treatment in excision method.
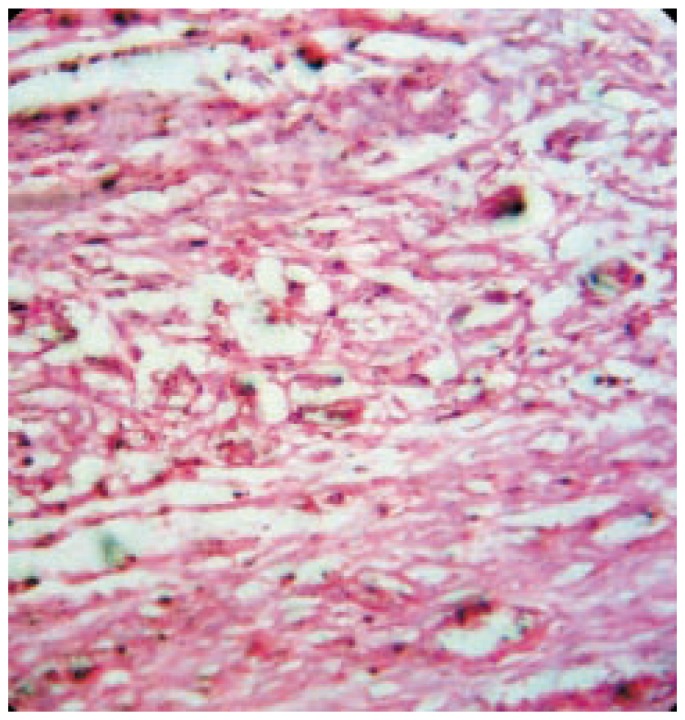
Hematoxylin and eosin stain (100×).
Figure 5. Photomicrograph of wound tissue without any treatment in excision method.
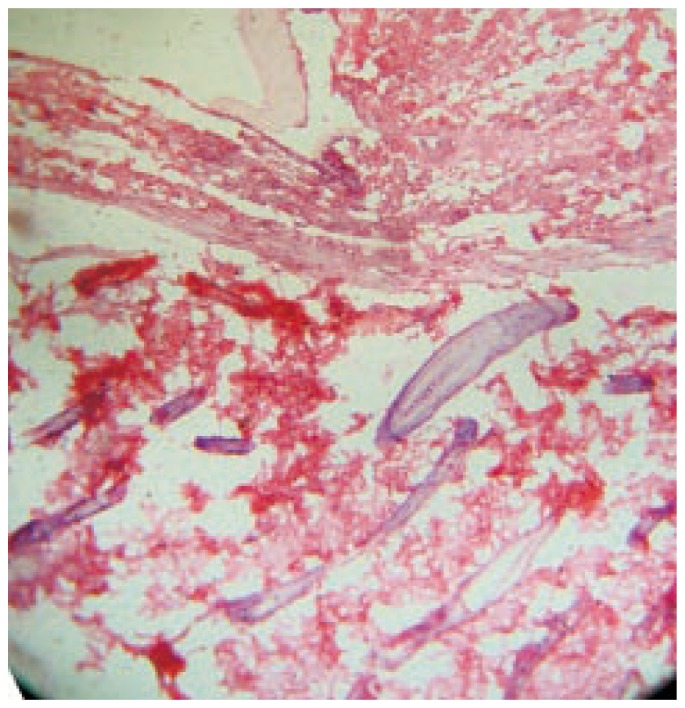
Hematoxylin and eosin stain (100×).
Figure 6. Photomicrograph of wound tissue with MAF-A fraction treatment in excision method.
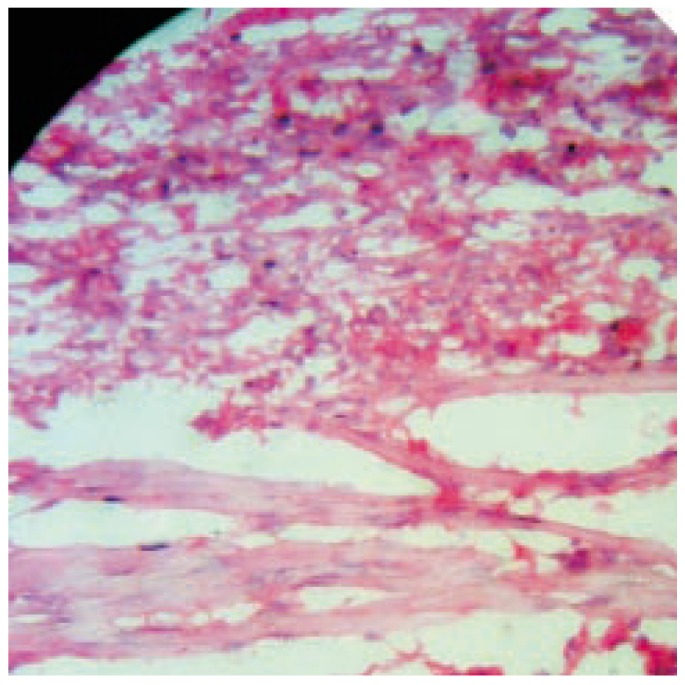
Hematoxylin and eosin stain (100×).
Figure 7. Photomicrograph of wound tissue with MAF-B fraction treatment in excision method.
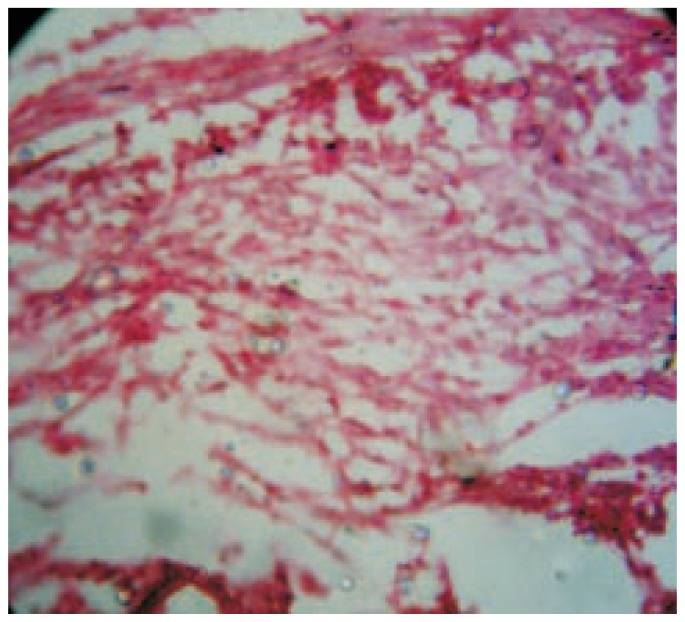
Hematoxylin and eosin stain (100×).
4. Discussion
Wound healing is a complex process that results in wound contraction, wound closure and restoration of the functional barrier. Cutaneous wound repair is accompanied by a definite ordered and sequence of biological events starting with wound closure and progressing to the repair and remodeling of damaged tissue[16].
The aim of this work was to evaluate wound healing property of M. annua Linn leaves extract using two wound models. Epithelialization period was decreased as a result of treatment with different fractions of M. annua Linn. Additionally, there was a reduced visible scar area. There was a significant increase in the tensile strength and hydroxyproline content compared with control group and comparable to reference group (P<0.01). The observation and results in this study indicate that MAF-C fraction of M. annua Linn. may significantly stimulate wound contraction as well as epithelialization.
Phytochemical work demonstrates that ethanol extract of M. annua Linn leaves contains flavonoids, which have been documented to have antioxidant, free radical scavenging effect and antibacterial activity[17]. As a result of chromatographic study, the MAF-C fraction contains luteolin, that is well reported to have antioxidant effect[18]. MAF-C fraction also gives positive Shinoda test of flavonoids. Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a flavonoid widely distributed in the plant kingdom, showing a concentration-dependent inhibitory activity in several models of oxidative stress[19]. The antioxidant potential of luteolin, is twice stronger than that of vitamin E. Luteolin also has strong scavenging properties for superoxide radicals. It is a potent physical quencher of singlet oxygen. Luteolin inhibits single strand break in DNA induced by singlet oxygen in a dose-dependent manner[20],[21]. The antioxidant activity of methanolic and aqueous extracts of M. annua Linn. leaves had already been reported[22].
Collagen is a major protein of the extracellular matrix and the component that ultimately contributes to wound healing. Breakdown of collagen liberates free hydroxyproline and its peptides. Measurement of this hydroxyproline therefore has been used as an index of collagen turnover. The increased hydroxyproline content of the excision wound has indicated faster collagen turnover leading to rapid wound healing.
There is plenty of evidence to suggest that increased production of reactive oxygen species, lipid peroxidation and ineffective scavenging play a crucial role in various skin lesions and in modulation of fibroblast proliferation[23]. Cutaneous wounding causes a depression in the overall antioxidant status making it more vulnerable to oxygen radical attack[24]. All these findings indicate that in wound healing, antioxidants may play an important role.
In recent years naturally occurring herbal compounds such as phenolic acids, flavonoids, and high molecular weight polyphenols commonly known as tannins, are powerful antioxidants[25]. Flavonoids can prevent injury caused by free radicals in various ways. One way is the direct scavenging of free radicals. Flavonoids are oxidized by radicals, resulting in a more stable, less-reactive radical. Because of the high reactivity of the hydroxyl group of the flavonoids, radicals are made inactive. Selected flavonoids can directly scavenge superoxides, whereas other flavonoids can scavenge the highly reactive oxygenderived radical called peroxynitrite. By scavenging radicals, flavonoids can inhibit low density lipoproteins oxidation in vitro[26],[27]. Flavonoids have been shown to increase collagen synthesis, promote the cross-linking of collagen, decrease the degradation of soluble collagen, accelerate the conversion of soluble collagen to insoluble collagen, and inhibit the catabolism of soluble collagen. From a clinical point of view, collagen deposition in the wound is the most important phase of healing. Facilitating oxygen diffusion, increasing lymphatic drainage, diminishing oxygen free radical overproduction and increasing the collagen synthesis were together found to improve healing[28].
In conclusion, our findings suggest that flavonoid present in the fraction (MAF-C) of M. annua Linn. has a potential benefit in enhancing the wound healing process. This property may be due to free radical scavenging property of the plant which has been reported. It is suggested that further studies are required on human volunteers for a longer study period to thoroughly evaluate the efficacy of plant extract on different types of wounds.
Acknowledgments
The authors would like to acknowledge to Indian Council of Medical Research, New Delhi for financial support during research work.
Footnotes
Foundation Project: Supported by Indian Council of Medical Research, New Delhi India (No. 2007-00690).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Falabella AF, Kirsner RS. Wound healing: historical aspects of wound healing. Boca Raton: Taylor & Francis Group; 2005. p. 1. [Google Scholar]
- 2.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 3.Biswas TK, Maity LN, Mukherjee B. Wound healing potential of Pterocarpus santalinus Linn: a pharmacological evaluation. Int J Low Extrem Wounds. 2004;3(3):143–150. doi: 10.1177/1534734604268385. [DOI] [PubMed] [Google Scholar]
- 4.Manandhar NP, Manandhar S. Plants and people of Nepal. Portland: Timber Press, Inc; 2002. p. 311. [Google Scholar]
- 5.Khare CP. Indian medicinal plants: an illustrated dictionary. Springer-Verlag Heidelberg; 2007. pp. 399–400. [Google Scholar]
- 6.Mali PC, Ansari AS, Chaturvedi M. Antifertility effect of chronically administered Martynia annua root extract on male rats. J Ethnopharmacol. 2002;82(2–3):61–67. doi: 10.1016/s0378-8741(02)00084-3. [DOI] [PubMed] [Google Scholar]
- 7.Chatpalliwar VA, Joharapurkar AA, Wanjari MM, Chakraborty RR, Kharkar VT. Antiinflammatory activity of Martynia diandra GLOX. Indian Drugs. 2002;39(10):543–545. [Google Scholar]
- 8.Sadaf F, Saleem R, Ahmed M, Ahmad SI, Zafar N. Healing potential of cream containing extract of Sphaeranthus indicus on dermal wounds in Guinea pigs. J Ethnopharmacol. 2006;107(2):161–163. doi: 10.1016/j.jep.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Kumari M, Eesha BR, Amberkar M, babu S, Rajshekar KN. Wound healing activity of aqueous extract of Crotalaria verrucosa Wistar albino rats. Asian Pac J Trop Med. 2010;3(10):783–787. [Google Scholar]
- 10.Silambujanaki P, Chandra CT, Kumar KA, Chitra V. Wound healing activity of Glycosmis arborea leaf extract in rats. J Ethnopharmacol. 2010;134(1):198–201. doi: 10.1016/j.jep.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 11.Rashed AN, Afifi FU, Disi AM. Simple evaluation of wound healing activity of a crude extract of Portuloca oleracea Linn. (growing in Jorden) in Mus musculus JVI-1. J Ethanopharmacol. 2003;88:131–136. doi: 10.1016/s0378-8741(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 12.Kuwano H, Yano K, Ohano S, Lkebe M, Kitamura K, Toh Y, et al. et al. Dipyridamole inhibits early wound healing in rat skin incisions. J Surg Res. 1994;56:267–270. doi: 10.1006/jsre.1994.1042. [DOI] [PubMed] [Google Scholar]
- 13.Bancroft JD, Gamble M. Theory and practice of histological techniques. 6th ed. Churchill Livingstone Elsevier; 2008. pp. 93–133. [Google Scholar]
- 14.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small portion of this imino acid. Arch Biochem Biophys. 1961;193:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 16.Rajan RG, Kumar MV, Rao CV, Shirwaikar A, Mehrotra S, Pushpangadan P. Healing potential of Anogeissus latifolia for dermal wounds in rats. Acta Pharm. 2004;54:331–338. [PubMed] [Google Scholar]
- 17.Sengar NPS, Pathak AK, Mehta PD, Shirode D, Singh A. Comparative antimicrobial studies of ethanolic extract of leaves of Nerium indicum and Martynia annua. Biosci Biotechnol Res Asia. 2006;3(1) [Google Scholar]
- 18.Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 19.Cotelle N, Bernier JL, Catteau JP, Pommery J, Wallet JC, Gaydou EM. Antioxidant properties of hydroxy-flavones. Free Radic Biol Med. 1996;20:35–43. doi: 10.1016/0891-5849(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 20.Kumpulainen JT, Salonen JT. Natural antioxidants and food quality in atherosclerosis and cancer prevention. Cambridge: The Royal Society of Chemistry; 1996. pp. 56–259. [Google Scholar]
- 21.Tournaire C, Croux S, Maurette MT, Beck I, Hocquaux M, Braun AM, et al. et al. Antioxidant activity of flavonoids: efficiency of singlet oxygen quenching. J Photochem Photobio B. 1993;19:205–215. doi: 10.1016/1011-1344(93)87086-3. [DOI] [PubMed] [Google Scholar]
- 22.Nagda D, Saluja A, Nagda C. Antioxidant activities of methanolic and aqueous extracts from leaves of Martynia annua Linn. Pharmacogn J. 2009;1(4):288–287. [Google Scholar]
- 23.Murrell GAC, Francis MJO, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla A, Rasik AM, Patnaik GK. Depletion of reduced glutathione, ascorbic acid, vitamin E and antioxidant defense enzymes in a healing cutaneous wound. Free Radic Res. 1997;26:93–101. doi: 10.3109/10715769709097788. [DOI] [PubMed] [Google Scholar]
- 25.Svobodova A, Psotova J, Walterova D. Natural phenolics in the prevention of Uv-induced skin damage: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;47(2):137–145. [PubMed] [Google Scholar]
- 26.Nijveldt RJ, Nood E, Hoorn DE, Boelens PG, Norren K, Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 27.Kumari M, Eesha BR, Amberkar M, Babu S, Rajshekar, Kumar N. Wound healing activity of aqueous extract of Crotalaria verrucosa in Wistar albino rats. Asian Pac J Trop Med. 2010;3(10):783–787. [Google Scholar]
- 28.Inan A, Sen M, Koca C, Akpinar A, Dener C. The effect of purified micronized flavonoid fraction on the healing of anastomoses in the colon in rats. Surg Today. 2006;36:818–822. doi: 10.1007/s00595-006-3251-4. [DOI] [PubMed] [Google Scholar]