Abstract
Objective
To determine the level of oxygen-nitrogen stress parameters in the pathogenesis of amebiasis.
Methods
Twenty-four acute intestinal amebiasis patients and 20 healthy controls were enrolled in the present study. Serum malondialdehyde and nitric oxide levels were determined spectrophotometrically.
Results
Serum malondialdehyde and nitric oxide levels were significantly higher in acute intestinal amebiasis patients than healthy controls (P<0.001).
Conclusions
These results suggest that oxidative and nitrosative stress may play a major role in tissue damage in acute intestinal amebiasis patients. Also these parameters can be used to supplement the conventional microscopic method for reliable diagnosis of intestinal amebiasis.
Keywords: Amebiasis, Malondialdehyde, Nitric oxide
1. Introduction
Entamoeba histolytica (E. histolytica) is the third leading parasitic cause of death in developing countries and one of the important health risks to which travelers are exposed. It is estimated that more than 10% of the world's population are infected by E. histolytica[1].
Human amebiasis is the infection of the human gastrointestinal tract by E. histolytica, a protozoan parasite that is well known for its high potential for invading and destroying human tissue, leading to diseases such as hemorrhagic colitis and extraintestinal abscesses[2]–[4]. The high prevalence and mortality of this disease is of interest because they raise several questions regarding the nature of amebiasis and the capacity of the host to mount defenses against the parasite[5].
The parasite usually lives and multiplies within the human gut, which constitutes an environment of reduced oxygen pressure. During tissue invasion, E. histolytica is exposed to elevated amounts of exogenous reactive oxygen species (ROS), such as superoxide radical anions (O2−) and hydrogen peroxide (H2O2). Additionally, the parasite can stimulate the activation of the host's immune response, which results in production of the cytokines TNF-α and IFN-α, and these increase respiratory burst (and ROS) on phagocytes[6]–[8]. These highly toxic molecules cause severe damage to biological macromolecules (such as lipids, proteins and DNA) leading to metabolic malfunctions[9]. Lipid peroxidation reflects the interaction between ROS and polyunsaturated fatty acids, and induces oxidation of various breakdown products of the latter. Among these, malondialdehyde (MDA) is a reliable marker of oxidative damage.
The clinical spectrum of the disease is due to the severity of the immune response of the host. The cellular immune response against the disease is fundamental and vitally important. Until the last decade, it was believed that free oxygen radicals were the most important part of the cellular immune response involved in killing the parasite. However, some studies demonstrated that reactive nitrogen radicals represent the main mechanism in the elimination of E. histolytica from the body[10]. The low polarity of nitric oxide (NO) allows it to freely diffuse through membranes. Damage to lipids does not only affect metabolic processes, but more importantly may also result in cell membrane permeability[11].
NO produced by activated macrophages is the major cytotoxic molecule for in vitro cytotoxicity against E. histolytica trophozoites[12],[13].
Therefore, a significant contribution to E. histolytica's pathogenic potential is likely to be due to its ability to cope with oxidative and nitrosative stresses generated during tissue invasion. To our knowledge, this is the first study conducted to evaluate serum MDA, which is an indicator of lipid peroxidation and a NO level marker of nitrosative stress in patients with acute intestinal amebiasis.
2. Materials and methods
2.1. Subjects
This study was conducted at Gaziantep University, Faculty of Medicine, Department of Infections Diseases and Department of Biochemistry and Clinical Biochemistry. The study protocol was carried out in accordance with the Helsinki declaration as revised in Tokyo 2004. All participants were informed about the study protocol and written consent was obtained from each of them. The local ethics committee approved the study protocol.
Twenty four patients (14 men / 10 women; mean age ±SEM, 29.4±1.5 years; range: 16-45) with acute E. histolytica rectocolitis that were otherwise healthy were enrolled in this study. Amebiasis cases were enrolled over a three week period and a control group was enrolled over a week. Diagnosis of amebiasis was made by identifying trophozoites in fresh stool specimens and preparations stained with trichrome by microscopic examination. Twenty healthy subjects (12 men / 8 women; mean age±SEM, 29.8±2.0 years; range: 16-47) who were referred to the out patient clinic for routine check-up purposes were recruited to the control group.
All participants were monitored and excluded if symptoms of infection (other than E. histolytica rectocolitis) or systemic somatic illness were present. History of alcohol abuse and smoking also led to exclusion from the study.
Fasting venous blood samples were collected using standard venipuncture technique between 9:30-11:00 am after 12 h fast. Sera were seperated immediately by centrifugation at 1 000 g for 10 min and stored at -70 °C until analysis, which was performed in the same run. Haemolyzed specimens were excluded.
2.2. Measurement of total plasma nitrite level
NO is a labile compound and has a brief half-life, and therefore its detection as the native NO molecule is difficult. It is rapidly converted to the stable end-products nitrate (NO3−) and nitrite (NO2−) in typical oxygenated aqueous solutions and tissues. Thus, plasma total nitrite levels were measured as an index of NO production. For total nitrite detection, 300 µL of plasma was deproteinized by adding 600 µL of 75 mmol/L ZnSO4 solution, stirring, and centrifuging at 1 000 g for at least 5 min at room temperature, after which 600 µL of 55 mmol/L NaOH was added. Total nitrite was quantitated by means of the Griess reaction after incubation of plasma samples with copperized cadmium (Cd) granules for 90 min to convert NO3− to NO2− in glisin-NaOH buffer (pH = 9.7). Griess reagent (1 mL 0.5% sulphanilamide and 0.05% N-naphthyletilene diamine hydrochloride) was then added to 1 mL of plasma specimens. Absorbance was read at 545 nm after a 30 min incubation in the dark. Standard curves were prepared with known concentrations (1-100 µmol/L) of sodium nitrite. The results were given in µmol/L.
2.3. Measurement of serum malondialdehyde levels
MDA analysis is a method based on the reaction of thiobarbituric acid with some products of lipid peroxidation in an acidic environment at an increased temperature. The product forms a pink colour which enables its spectrophotometric determination. The determination procedure consists of the addition of 1.5 mL of 20% acetic acid, 1.5 mL of 0.8% thiobarbituric acid, 200 µL of 8.1% sodium dodecil sulphate, and 700 µL of distilled water to a 100 µL sample. The reaction mixture was heated in boiling water for 60 min. After cooling, 1 mL of distilled water and 5 mL of buthanol/pyridine (14/1: v/v) were added to the samples. The samples were then centrifuged at 900 g for 10 min. Absorbance of the supernatant was measured at 532 nm. The MDA concentration was calculated according to a standard curve prepared from 1,1,3,3-tetramethoxypropane.
2.4. Statistical analysis
Results were statistically analysed using the analysis of variance or the Mann-Whitney U-test as indicated, and expressed as mean ± standard error of mean (SEM). P<0.05 was considered a significant difference between groups. Statistical analysis was performed with the Statistical Package for the Social Sciences for Windows (version 11.5; SPSS Inc., Chicago, IL, USA).
3. Results
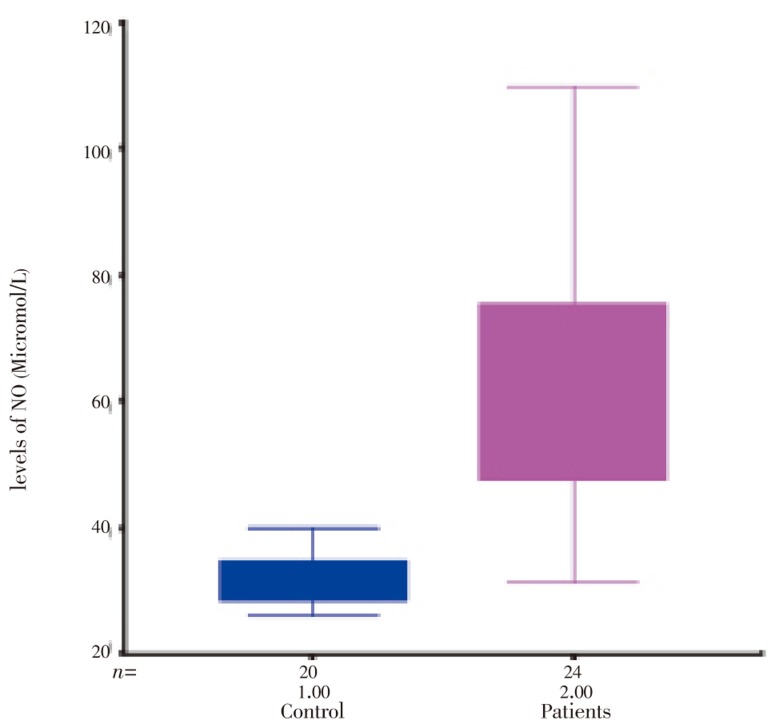
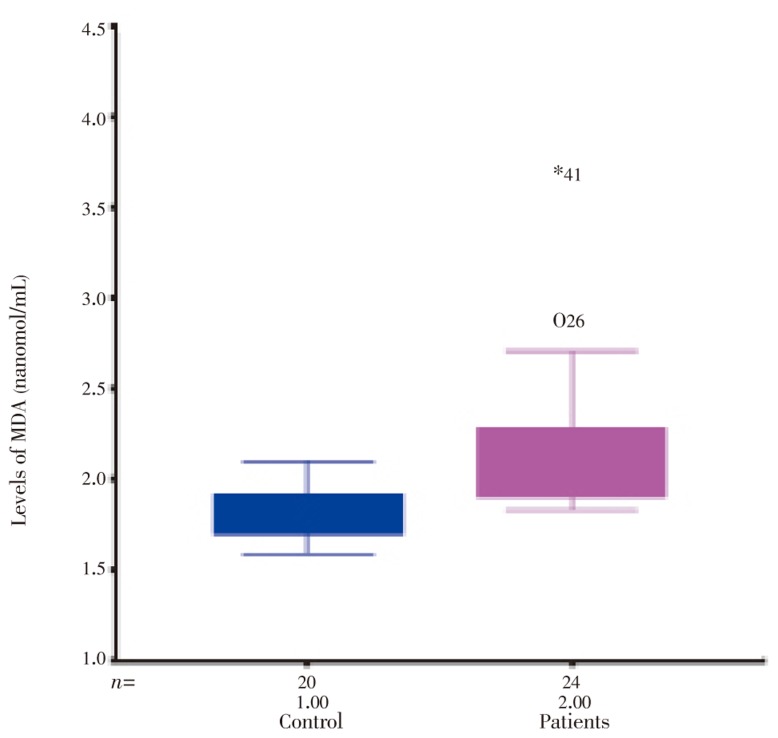
As shown in Table 1 the patients and controls were similar in age/gender. MDA and NO were significantly increased in patients with acute intestinal amebiasis compared with controls (P<0.001) ( Table 1, Figure 1 and 2).
Table 1. The demograpfic data and serum levels of MDA, NO in patients with acute intestinal amebiasis and healthy controls.
| Parameter | Healthy control (n=20) | Patients with amebiasis (n=24) |
| Age (year) | 29.8±2.0 | 29.4±1.5 |
| Gender | 12M / 8F | 14M / 10F |
| MDA (nanomol/mL) | 1.80±0.03 | 2.22±0.10* |
| NO (µmol/L) | 31.55±0.94 | 62.08±3.84* |
*P<0.001; M: Male; F: Female.
Figure 1. The levels of NO in healthy control and patients with acute Intestinal amebiasis.
Figure 2. The levels of MDA in healthy control and patients with acute intestinal amebiasis.
4. Disscussion
In clinical and experimental E. histolytica infections, macrophage-mediated effector mechanisms have been shown to be important in control the resistance to reinfection[15]. Upon invasion of the host intestinal epithelium, E. histolytica trophozoites are confronted with varying oxygen tensions and cytotoxic reactive oxygen and nitrogen species[15].
Gut inflamation is seen in individuals with amoebic colitis. Intestinal epithelial cells are important sensors of microbial infection. Intestinally derived transformed cell lines produce a variety of pro-inflammatory mediators in response to intracellular infection with bacteria including interleukins 1 and 8 cyclooxygenase-2 and inducible nitric oxide synthase[16]–[21]. Several of these mediators cause activation of neutrophils and macrophages, providing a mechanism by which intestinal epithelial cells import these cells to the site of pathogen invasion. The clearance of E. histolytica trophozoites by neutrophils and macrophages is contact dependent and involves both oxidative and nonoxidative pathways[6]–[8].
Reactive oxygen species are highly reactive. When they are generated close to cell membranes, possibly by intestinal epithelial cells, they induce oxidative stress and oxidized membrane phospholipids (lipid peroxidation), which might continue in the form of a chain reaction. Free radicals can attack polyunsaturated fatty acids in biomembranes, because they are susceptible to oxidative stress. Polyunsaturated fatty acids of cell membranes are degraded by lipid peroxidation with subsequent disruption of membrane integrity, suggesting that lipid peroxidation mediated by oxygen free radicals is an important cause of damage and destruction of cell membranes[22]. We have shown that MDA an index of lipid peroxidation, significantly increased in patients with acute intestinal amebiasis compared with the control group (P<0.001).
In addition to direct products derived from activated neutrophils, the secondary products induced by oxidative stress may play a role in the development of intestinal inflammation. Experimental and clinical evidence have suggested that MDA, a product during lipid peroxidation, can act as a bioactive molecule in either physiological or pathological conditions. As a result, lipid peroxidation causes changes in membrane permeability and selectivity and ultimately leads to alterations in cell volume homeostasis and cellular metabolism. Moreover, hydroperoxides and aldehydes are directly toxic to cells and organelles, have neutrophil chemotactic properties and may regulate cytokine production[16]–[22].
In recent years, considerable interest has been shown in the role of reactive nitrogen molecules in cellular redox reactions. Although NO is a highly lipid-soluble molecule with a considerably long high life, capable of diffusing through several cells from its site of synthesis, there is still much debate as to whether NO production is actually harmful to inflamed tissue or not. It is also unclear whether the NO producing enzyme nitric oxide synthase, is expressed by inflammatory cells[16]–[21]. We have found that NO an index of nitrosative stress, is significantly increased in patients with acute intestinal amebiasis comparing with the control subjects (P<0.001). This is the first study in acute intestinal amebiasis patients, that showed an increase in lipid peroxidation and nitrosative stress.
The diagnosis of amebiasis is frequently dependent on the sequential examination of several faecal samples by a skilled person. In many cases, the parasite is not revealed by examination of a single faecal sample. Hiatt et al recommended that three samples per patient should be examined; otherwise the diagnosis rate might be significantly underestimated. According to these authors, the diagnostic yield increased 22.7% for E. histolytica, when three samples were examined instead of only one[22].
In conclusion, our findings of increased levels of serum MDA and NO in the acute intestinal amebiasis group supports the hypothesis of an increase of free radical levels in these patients. Activated neutrophils may produce reactive oxygen and nitrogen species within intestinal mucosa, which induce oxidative stress. Recent clinical data suggest that reactive oxygen/nitrogen metabolite-mediated injury is important in both primary and downstream secondary pathophysiological mechanisms underlying intestinal inflammation. At the same time these parameters can be used to supplement the conventional microscopic method for reliable diagnosis of intestinal amebiasis especially in cases with low parasitaemia. Further investigations are required to elucidate the relationship of these changes with the pathogenesis of inflammation in acute intestinal amebiasis.
Footnotes
Foundation Project: Supported by Scientific Research Projects Governing Unit of Gaziantep University (No. TF: 00 04).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ravdin JI. Entamoeba histolytica. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious disease. Newyork: E-publishing Churchill Livingstone Inc; 2000. pp. 2798–2810. [Google Scholar]
- 2.Villalba JD, Gómez C, Medel O, Sánchez V, Carrero JC, Shibayama M, et al. et al. Programmed cell death in Entamoeba histolytica induced by the aminoglycoside G418. Microbiology. 2007;153(11):3852–3863. doi: 10.1099/mic.0.2007/008599-0. [DOI] [PubMed] [Google Scholar]
- 3.Anaya-Velázquez F, Padilla-Vaca F. Virulence of Entamoeba histolytica: a challenge for human health research. Future Microbiol. 2011;6:255–258. doi: 10.2217/fmb.11.2. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Palomo A, Espinosa-Cantellano M. Amoebiasis: new understanding and new goals. Parasitol Today. 1998;14:1–3. doi: 10.1016/s0169-4758(97)01176-9. [DOI] [PubMed] [Google Scholar]
- 5.Franca-Botelho AC, Franca JL, Oliveira FM, Franca EL, Honorio-Franca AC, Caliari MV, et al. et al. Melatonin reduces the severity of experimental amoebiasis. Parasit Vectors. 2011;4(1):62–69. doi: 10.1186/1756-3305-4-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh AS, Dutta S, Raha S. Hydrogen peroxide-induced apoptosis-like cell death in Entamoeba histolytica. Parasitol Int. 2010;59(2):166–172. doi: 10.1016/j.parint.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford: Oxford University Press; 2007. pp. 377–388. [Google Scholar]
- 8.Stanley SL. Amebiasis. Lancet. 2003;361:1025–1034. doi: 10.1016/S0140-6736(03)12830-9. [DOI] [PubMed] [Google Scholar]
- 9.Sen A, Chatterjee NS, Akbar MA, Nandi N, Das P. The 29-kilodalton thiol-dependent peroxidase of Entamoeba histolytica is a factor involved in pathogenesis and survival of the parasite during oxidative stress. Eukaryot Cell. 2007;6:664–673. doi: 10.1128/EC.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chikobava GI, Sanikidze TV. The role of oxygen-nitrogen stress in pathogenesis of amoebiasis. Georgian Med News. 2006;(131):96–99. [PubMed] [Google Scholar]
- 11.Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans. 2007;35:1147–1150. doi: 10.1042/BST0351147. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez-Emiliano J, Flores-Villavicencio LL, Segovia J, Arias-Negrete S. Nitric oxide participation during amoebic liver abscess development. Medicina (B Aires) 2007;67(2):167–176. [PubMed] [Google Scholar]
- 13.Olivos-García A, Saavedra E, Ramos-Martínez E, Nequiz M, Pérez-Tamayo R. Molecular nature of virulence in Entamoeba histolytica. Infect Genet Evol. 2009;9(6):1033–1037. doi: 10.1016/j.meegid.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Vicente JB, Ehrenkaufer GM, Saraiva LM, Teixeira M, Singh U. Entamoeba histolytica modulates a complex repertoire of novel genes in response to oxidative and nitrosative stresses: implications for amebic pathogenesis. Cell Microbiol. 2009;11:51–69. doi: 10.1111/j.1462-5822.2008.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naito Y, Tukagi T, Yoshikawa T. Neutrophil-dependent oxidative stress in ulcerative colitis. J Clin Biochem Nutr. 2007;41:18–26. doi: 10.3164/jcbn.2007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad N, Fazal H, Abbasi BH, Iqbal M. In vitro larvicidal potential against Anopheles stephensi and antioxidative enzyme activities of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorous. Asian Pac J Trop Med. 2011;4(3):169–175. doi: 10.1016/S1995-7645(11)60063-1. [DOI] [PubMed] [Google Scholar]
- 18.Nwagha UI, Okeke TC, Nwagha TU, Ejezie FE, Ogbodo SO, Dim CC, et al. et al. Asymptomatic malaria parasitemia does not induce additional oxidative stress in pregnant women of South East Nigeria. Asian Pac J Trop Med. 2011;4(3):229–233. doi: 10.1016/S1995-7645(11)60076-X. [DOI] [PubMed] [Google Scholar]
- 19.Karthikeyan R, Somasundaram ST, Manivasagam T, Balasubramanian T, Anantharaman P. Hepatoprotective activity of brown alga Padina boergesenii against CCl4 induced oxidative damage in Wistar rats. Asian Pac J Trop Med. 2010;3(9):696–701. [Google Scholar]
- 20.Rai B, Kaur J, Catalina M. Anti-oxidation actions of curcumin in two forms of bed rest: oxidative stress serum and salivary markers. Asian Pac J Trop Med. 2010;3(8):651–654. [Google Scholar]
- 21.Kumar A. Effect of simvastatin on paraoxonase 1 (PON1) activity and oxidative stress. Asian Pac J Trop Med. 2010;3(4):310–314. [Google Scholar]
- 22.Kruidenier L, Verspaget HW. Review article: oxidative stress as a pathogenic factor in inflammatory bowel disease-radical or ridiculous? Aliment Pharmacol Ther. 2002;16:1997–2015. doi: 10.1046/j.1365-2036.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 23.Hiatt RA, Markell EK, Ng E. How many stool examinations are necessary to detect pathogenic intestinal protozoa? Am J Trop Med Hyg. 1995;53(1):36–39. [PubMed] [Google Scholar]