Figure 1.
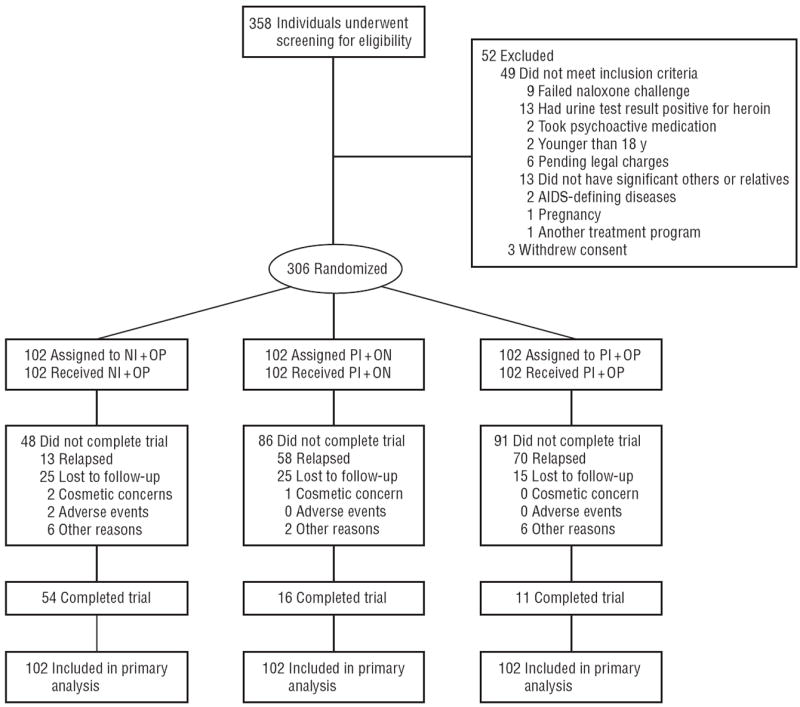
Study flow diagram. NI+OP indicates 1000-mg naltrexone implantandoral placebo; PI+NO, placebo implant and 50-mg oral naltrexone hydrochloride; PI+OP, placebo implant and oral placebo. The 2 adverse events in the NI+OP group include wound infection only.
