Abstract
Background
Anti-Galα1,3Galβ-R natural antibodies are responsible for hyperacute rejection in pig-to-primate xenotransplantation. Although the generation of pigs lacking the α1,3galactosyltransferase (GalT) has overcome hyperacute rejection, antibody-mediated rejection is still a problem. It is possible that other enzymes synthesize antigens similar to Galα1,3Gal epitopes that are recognized by xenoreactive antibodies. The glycosphingolipid isoglobotrihexosylceramide (iGb3) represents such a candidate expressing an alternative Galα1,3Gal epitope. The present work determined whether the terminal Galα1,3Gal disaccharide is completely absent in Immerge pigs lacking the GalT using several different highly sensitive methods.
Methods
The expression of Galα1,3Gal was evaluated using a panel of antibodies and lectins by flow cytometry and fluorescent microscopy; GalT activity was detected by an enzymatic assay; and ion trap mass spectroscopy of neutral cellular membranes extracted from aortic endothelial was used for the detection of sugar structures. Finally, the presence of iGb3 synthase mRNA was tested by RT-PCR in pig thymus, spleen, lymph node, kidney, lung, and liver tissue samples.
Results
Aortic endothelial cells derived from GalT knockout pigs expressed neither Galα1,3Gal nor iGb3 on their surface, and GalT enzymatic activity was also absent. Lectin staining showed an increase in the blood group H-type sugar structures present in GalT knockout cells as compared to wild-type pig aortic endothelial cells (PAEC). Mass spectroscopic analysis did not reveal Galα1,3Gal in membranes of GalT knockout PAEC; iGb3 was also totally absent, whereas a fucosylated form of iGb3 was detected at low levels in both pig aortic endothelial cell extracts. Isoglobotrihexosylceramide 3 synthase mRNA was expressed in all pig tissues tested whether derived from wild-type or GalT knockout animals.
Conclusions
These results confirm unequivocally the absence of terminal Galα1,3Gal disaccharides in GalT knockout endothelial cells. Future work will have to focus on other mechanisms responsible for xenograft rejection, in particular non-Galα1,3Gal antibodies and cellular responses.
Keywords: α1, 3galactosyltransferase, isoglobotrihexosylceramide, knockout pigs, transplantation, xenoantigen
Introduction
Natural antibodies (Ab) are responsible for hyperacute rejection in pig-to-primate xenotransplantation [1,2]. The main target of these natural Ab is the disaccharide Galα1,3Galβ-R (αGal), which is mainly synthesized by α1,3galactosyltransferase (GalT) [3], an enzyme found in all mammals, except in higher primates and humans [4]. The generation of pigs with disrupted GalT by knocking out the GalT gene (GalT KO) has overcome the hurdle of hyperacute rejection [5,6]. However, after the generation of GalT KO animals, there was a controversy about the complete elimination of αGal epitopes coming from positive staining for αGal epitope in GalT KO and the discovery of a new enzyme able to add αGal to lipids [7]. A possible explanation for the reported residual expression of αGal on GalT KO pigs is provided by the findings in GalT KO mice and rats of a second enzyme able to synthesize αGal, the isoglobotrihexosylceramide synthase (iGb3S) [8,9]. The latter belongs to the family of ABO-blood group glycosyltransferases and initiates the synthesis of isoglobo-series of glycosphingolipids [10]. In contrast to GalT, iGb3S uses the common precursor lactosylceramide (LacCer) as substrate [9,10], suggesting the possibility of alternative pathways for the synthesis of αGal epitopes and, therefore, their expression in GalT KO pigs. The aim of the present work was to confirm the absence of the terminal Galα1,3Gal disaccharide in GalT KO pigs in comprehensive methodological approach using simultaneously different reagents, including a nearly complete panel of available monoclonal anti-Gal antibodies, and highly sensitive techniques such as ion trap mass spectroscopy (MS) to evaluate αGal expression, a novel enzymatic assay for GalT, and RT-PCR for iGb3 synthase.
Material and methods
Cell lines and tissues
Porcine aortic endothelial cells isolated from wildtype (PAEC WT) and PAEC derived from GalT knockout pigs (PAEC GalT KO) [11] were kindly provided by R.J. Hawley (former Immerge Biotherapeutics, Cambridge, MA, USA). Thymus, spleen, lymph node, kidney, lung, and liver tissues from both WT and GalT KO miniature swine were kindly provided by D. Sachs and J. Hanekamp (Massachusetts General Hospital, Boston, MA, USA) [11]. Two different samples from each organ were obtained from a WT animal (#16517, female, AA haplotype, 2.5 yr old) and a GalT KO animal (#16183, female, DD haplotype, 3.5 yr old), respectively. Tissue samples were snap frozen in a RNA stabilization solution and stored at −80 °C until RNA extraction and mRNA analysis. The human embryonic kidney cell line E293 transfected with rat GalT was a kind gift from M.S. Sandrin (University of Melbourne Department of Surgery, Austin Health, Heidelberg, Victoria, Australia). All cell lines were cultured as previously reported [12,13].
Anti-αGal and H-type structures reagents
For the detection of αGal epitopes on the cell surface, the following antibodies and lectins were used: (i) human polyclonal anti-αGal antibodies previously generated by affinity purification and characterized in our laboratory [14]; (ii) mouse monoclonal Ab (mAb) anti-αGal IgM M86 (Alexis Corporation, Lausen, Switzerland); (iii) FITC-conjugated isolectin B4 from Bandeiraea simplicifolia (BS-IB4; Sigma, Buchs, Switzerland); (iv) four different murine mAb: 15.101, 25.2, 24.7, and 22.121, which were a kind gift from M.S. Sandrin [13,15] and thoroughly described previously [15]; (v) two different clones of murine mAb clones GT4-31 and GT6-27, which were a kind gift from A.S. Chong (University of Chicago, Chicago, IL, USA) [16]; and the murine mAb 4F10 that was a gift of A. Bendelac (Howard Hughes Medical Institute and University of Chicago, Chicago, IL, USA). Additionally, the FITC-conjugated Ulex europaeus (UEA-I) lectin (Sigma) was used for flow cytometry.
Flow cytometry
Surface expression of the αGal epitope and blood group H-type structures on PAEC WT and GalT KO cells was analyzed using a FACSCanto (Becton Dickinson, Basel, Switzerland) by direct and indirect immunofluorescence using the above-mentioned reagents. Cells were incubated for 30 min at 4 °C with saturating amounts of these reagents. Goat anti-mouse IgM/IgG FITC-conjugated (BD-Pharmingen, Basel, Switzerland) was used as a secondary Ab and the following isotype-matched mAb as controls: mouse IgG1 (Sigma); mouse IgG3; and mouse IgM (BD-Pharmingen). For lectin staining, cells were incubated for 15 min at 4 °C. Propidium iodide staining was used to exclude dead cells. Data were analyzed with FlowJo® (TreeStar Inc, Ashland, OR, USA). To compare the levels of surface expression, the geometric mean fluorescence intensity ratios (MFIR) were calculated by dividing the mean fluorescence intensity of each sample with the mean fluorescence intensity of the isotype control mAb or staining buffer-only in the case of lectins.
Fluorescent microscopy
Both WT and GalT KO PAEC were grown in 96-well plates (Milian, Geneva, Switzerland) coated with bovine fibronectin (Sigma-Aldrich, Buchs, Switzerland). After 24 h, the monolayers were fixed with a cold acetone–ethanol solution (1: 2) for 10 min or with 80% acetone for 5 min at room temperature, respectively. Then, the monolayers were incubated with 50 μl of a buffer containing the different anti-αGal for 30 min at 37 °C; washed three times with PBS; and incubated at 37 °C for 30 min with 50 μl of buffer containing the respective secondary antibody. Finally, nuclei were counterstained with DAPI. After extensive rinsing, coverslips were mounted on glass slides using Fluokeep (Argene, Varilhes, France) and analyzed with a fluorescent IX71 microscope (Olympus, Hamburg, Germany).
Detection of iGb3 by multistep ion trap mass spectroscopy
A total of 108 cells were used to isolate neutral glycolipid membrane fractions of WT and GalT KO PAEC, which were permethylated as described by Li et al. [17]. The molecular ion profiles were obtained by linear ion trap mass spectrometry using the electrospray ionization mass spectroscopic method (LTQ-ESI-MS) as described [18]. Briefly, the iGb3 in isobaric mixture of Gb3 (Galα1,4Lac-Cer) and iGb3 standards was identified by comparing the different patterns of MS4 product ions from the sodiated molecular ions via the glycan fragment m/z 667 and the terminal disaccharide 1-ene ion m/z 445 (i.e., X → 667 → 445 →) of pure permethylated iGb3 standards via ESI-LIT-MS with those of permethylated Gb3. Characteristic fragment ions at MS4, 211, and 371 were used as evidence for the presence of iGb3.
α1,3galactosyltransferase assay
The GalT enzymatic assay was performed as described elsewhere [19–22] with slight modifications. Briefly, fresh cell extracts containing 1% Triton X-100 were prepared from PAEC WT, GalT KO, and E293 cells; and protein concentrations were determined with a BCA protein assay reagent (Pierce Chemical Co, Rockford, IL, USA). A typical reaction mixture contained 100 mM Tris (pH 7.0), 20 mM MnCl2, 101 μM UDP-Gal ([sim] 5000 cpm/nmol), mixture of UDP-[U-14C]Gal (Amersham Biosciences, Arlington Heights, IL, USA) and UDP-Gal (Calbiochem, Darmstadt, Germany), 500 mg asialofetuin, and 150 μg of protein stemming from the cell lysates. Controls without acceptor were assayed in parallel under the same conditions. After different incubation times at 37 °C, the enzymatic reaction was stopped by adding ice-cold 5% phosphotungstic acid. The precipitates were collected by suction filtration over GFA Whatman glass-microfiber filters (Millipore, Zug, Switzerland), washed with absolute alcohol, and dried. Finally, 14C-radioactivity was quantified in a Tri-Carb 2900TR liquid scintillation counter (Packard, Pangbourne, UK).
RT-PCR
Total RNA was isolated using Trizol (Invitrogen, Basel, Switzerland) and treated with DNase (Qiagen, Hombrechtikon, Switzerland) during RNA extraction to avoid possible genomic DNA contaminations. Before use, RNA quality was controlled on a 2100 Bioanalyzer (Agilent Technologies, Basel, Switzerland). Reverse transcription was achieved using ImProm II Reverse Transcriptase (Promega, Wallisellen, Switzerland) following the manufacturers protocol. Different sets of primers for pig iGb3S were tested, three primers already published: 5′ GGCGCTGGCAGGAC 3′, 5′ CGGCCAGCGGTAGTG 3′, 5′ CAGTGCGCCGTCAG 3′, named K2, K3, and K4, respectively [23], and the primers 5′ TCTTAGGGCTGCTCCTGTTTGG 3′ and 5′ AATGGTGAGGTTCTGCTGGGTAG 3′, named 67 and 68 correspondently, which were designed based on the iGb3S sequence reported in the published patents WO 2002081688 and WO 2005047469, respectively. Pig β2 microglobulin was used as housekeeping gene. All PCR products were visualized by agarose gel electrophoresis and cloned into a pGEM Easy vector (Promega) for further sequence analysis by using the flanking cloning site primers T7 and SP6. The sequence analysis was performed by Microsynth according to standards operating procedure (Microsynth, Balgach, Switzerland).
Results
Evaluation of αGal surface expression on pig endothelial cells
We first tested whether αGal was detectable on the cell surface of GalT KO PAEC using two different lectins and a panel of mAb directed against αGal. In addition, polyclonal human anti-αGal Abs were used as well as two mAb able to recognize iGb3 and the shared Galα1,3Gal disaccharide xenoantigen. The mAb 4F10, which recognizes both αGal and iGb3 (Fig. S1), unquestionably bound to WT but not to GalT KO PAEC, with a MFIR of 47.5 and 0.9, respectively (Fig. 1A). Similar results were obtained with the mAb 15.101, which has been reported to bind iGb3 and globotriaosylceramide (Gb3) [24], with a MFIR of 9.8 and 1.0 for WT and GalT KO PAEC, respectively (Fig. 1B). In addition, staining with M86; 25.2; 24.7; 22.121; GT4-31; and GT6-27 mAb, as well as polyclonal anti-αGal Ab isolated from human serum and the BSI-B4 lectin, confirmed the lack of αGal expression on GalT KO PAEC (Fig. 1C–I).
Fig. 1.
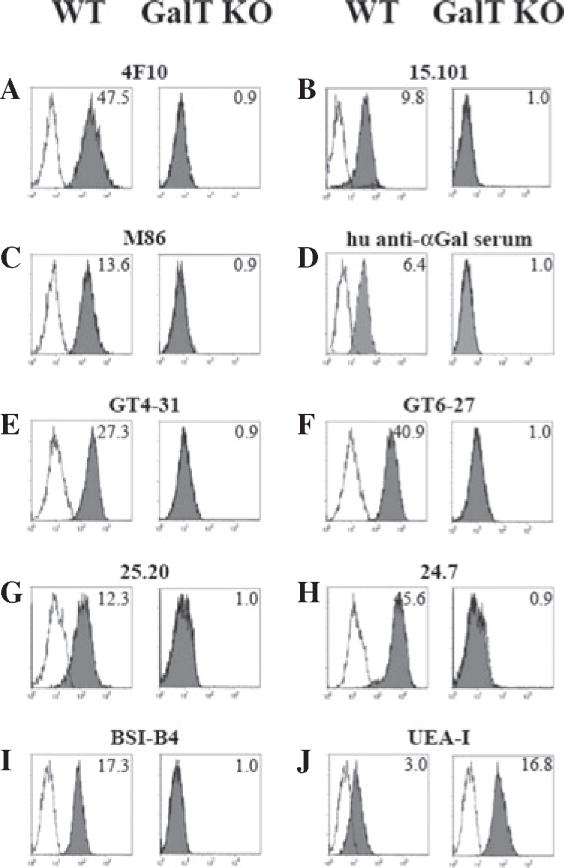
Lack of iGb3 and αGal expression on pig aortic endothelial cells derived from α1,3galactosyltransferase knockout pigs analyzed by flow cytometry. The epitopes iGb3 and αGal were tested in PAEC cells by indirect flow cytometry. Two mAb recognizing iGb3: clone 4F10 (A) with specificity for both αGal and iGb3 and clone 15.101 (B) were assayed in addition to the commercial mAb clone M86 (C); polyclonal purified anti-αGal from human serum (D); others mAb anti-αGal as the clone GT4-31 (E); clone GT6-27 (F); clone 25.20 (G); and clone 24.7 (H). Lectin staining with BSI-B4 (I) which binds to terminal α-D-galactosyl residues present in αGal and the UEA-I (J) which has with affinity for L-Fuc are also shown. Gray-shaded histograms show the staining for the different mAb, human serum, and lectins. Open histograms show the matching isotype controls when mAb were tested, human anti-αGal depleted serum for human serum, and cells incubated only with staining buffer for lectins staining. MFIR are shown in the upper right corner. Representative experiments from 3 to 5 different stainings are shown. BSI-B4, Bandeiraea simplicifolia lectin; GalT KO, α1,3galactosyltransferase knockout; iGb3, isoglobotrihexosylceramide 3; mAb, monoclonal antibodies; MFIR, Mean fluorescence intensity ratio; PAEC, pig aortic endothelial cell; UEA-I, Ulex europaeus lectin.
Staining of PAEC with UEA-I lectin that has a high affinity for L-fucose structures showed that GalT KO PAEC express higher levels of fucose structures in comparison with WT PAEC (MFIR, 16.8 vs. 3.0, respectively, Fig. 1J). To further determine the cellular distribution of the αGal epitope, fluorescent microscopic analysis was performed using the 4F10 and M86 mAb on PAEC cultured in 96-flat well plates. Figure 2 shows that there were no traces of αGal in GalT KO PAEC, whereas a clear and uniform labeling of cell PAEC WT staining was detected, thus confirming our flow cytometry results. In summary, the αGal epitope was not detected by antibody or lectin binding in GalT KO PAEC, neither by flow cytometry nor by fluorescent microscopy using a panel of seven different mAb, polyclonal anti-αGal Ab, and two lectins.
Fig. 2.
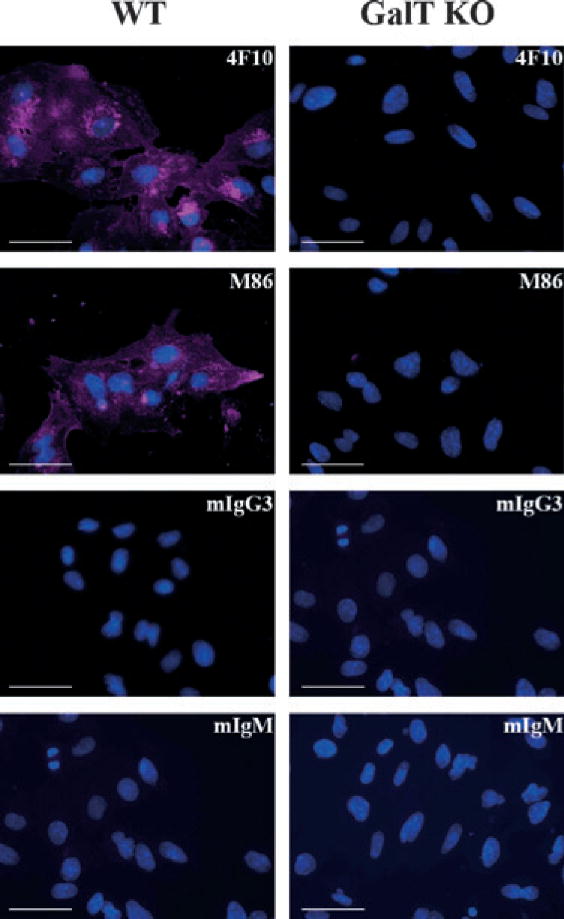
Uniform distribution of αGal in wild-type pig aortic endothelial cells. PAEC WT (left column) and GalT KO (right column) were grown directly in 96-well plates; fixed/permeabilized before staining with the 4F10 and M86 mAb or matching isotype controls: IgM and IgG3, respectively, as indicated in the top-right corner of the figures; and analyzed by Olympus fluorescent IX71 microscope fluorescent microscope. Overlay pictures of Evans blue channel in violet and DAPI channel in blue. Bar corresponds to 50 μm. Representative images of four different staining with the different pig cell lines. GalT KO, α1,3galactosyltransferase knockout; mAb, monoclonal antibodies; PAEC, pig aortic endothelial cell; WT, wild-type.
Mass spectroscopy analysis of αGal or iGb3 in cellular membranes of GalT KO PAEC
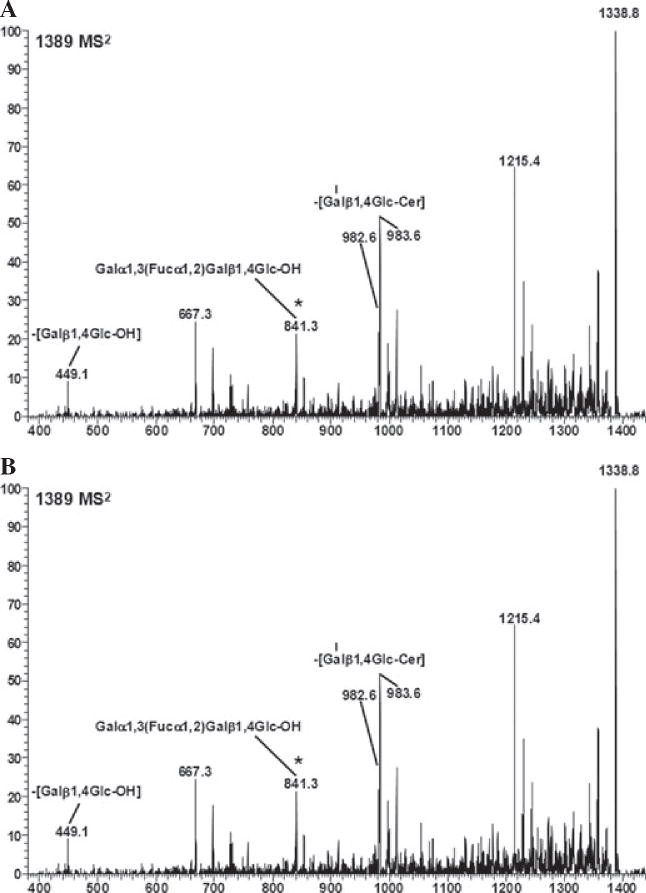
Next, we applied a highly sensitive MS technique to search for the presence of αGal carbohydrate structures in cellular membranes derived from PAEC. The mass spectroscopy 1 (MS1) analysis of neutral glycolipid fractions stemming from WT PAEC showed the presence of molecular ions located at 1664; 1748; and 1774 m/z, which correspond to fragments belonging to the terminal Galα1,3Gal disaccharide (specifically, Galα1,3Galβ1,4GlcβNAcβ1,3Galβ1,4Glc-Cer) (Fig. 3A). On the contrary, these ions were totally absent in GalT KO PAEC (Fig. 3B). In addition, the molecular ions of Gb4 (1460; 1486; 1514; 1544; and 1570 m/z); the Gb3 (1215; 1299, and 1325 m/z); and the precursor of iGb3, LacCer (1012 and 1120 m/z) were present in WT and GalT KO PAEC (Fig. 3). Because differences between Gb3 and iGb3 cannot be detected by MS1, sequential “breakdown” of the MS1 ions was performed by ion trap MS4 analysis (Fig. S2) [18] confirming that only Gb3 is present in both WT and GalT KO cells (data not shown). Additionally, we found the presence of a fucosylated-iGb3 structure in both WT and GalT KO PAEC, using the 841 m/z fragment by precursor ion mapping method (Fig. 4). However, the exact position of fucose in the tetrasaccharideceramide structure remains to be determined. Candidate structures are Galα1, 3(Fucα1,2)Galβ1,4Glc-Cer, or Fucα1,2Galα1, 3Galβ1, 4Glc-Cer. In addition, the precursor of the fucosylated- iGb3 (fucosylated LacCer) was found in WT PAEC (Fig. S3). Taken together, only WT PAEC cellular membranes express αGal bound to lipids, whereas Gb3 and its precursor LacCer are present in both WT and GalT KO PAEC, iGb3 is absent. A fucosylated-iGb3-type of structure was found in WT and GalT KO PAEC, but the position of fucose remains undetermined due its low abundance.
Fig. 3.
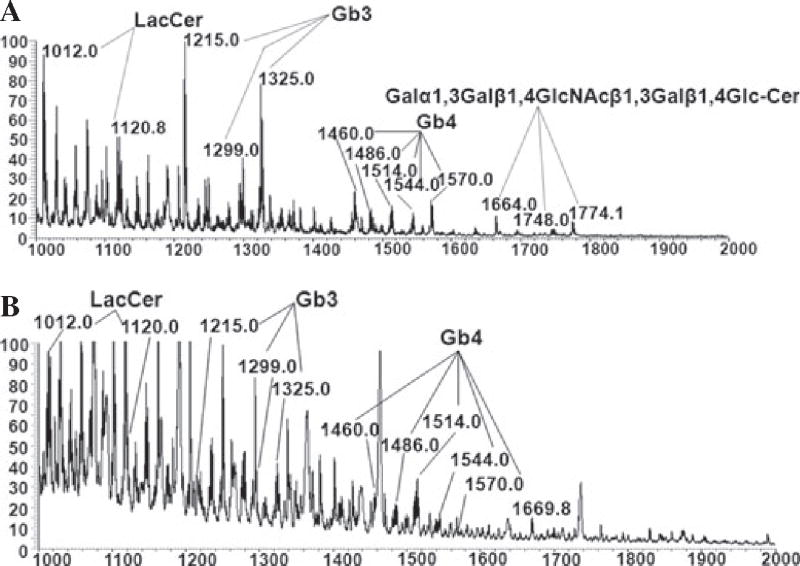
Absence of αGal and iGb3 in pig aortic endothelial cell derived from α1,3galactosyltransferase knockout pigs analyzed by electrospray ionization mass spectroscopy. Neutral glycosphingolipids membranes from PAEC were extracted, permethylated, and analyzed by MS. MS was carried out in positive ion mode on a linear ion trap mass spectrometer using a nano-electrospray source. MS1 neutral glycolipid fractions of WT (A) and GalT KO (B) PAEC were extracted and analyzed. Molecular ions containing αGal epitope (Galα3nLc4, Galα3Galβ4GlcNAcβ3Galβ4Glc-Cer) are only seen in WT PAEC (1664; 1748; and 1774 m/z). The molecular ions representing regioisomers of trihexosyceramides (Gb3/iGb3, 1215, 1299, and 1325 m/z) present in PAEC were further subjected to MS4 analysis by ion trap mass spectrometry excluding the presence of iGb3 [17]. The Gb3/iGb3 precursor, LacCer (1012 and 1120 m/z), is present in both PAEC Spectra correspond to x-axe in m/z and y-axe relative absorbance. Gb3, globotriaosylceramide also known as Pk antigen; iGb3, isoglobotrihexosylceramide; LacCer, lactosylceramide; PAEC, pig aortic endothelial cells; MS, mass spectroscopy.
Fig. 4.
Wild-type and α1,3galactosyltransferase knockout neutral pig aortic endothelial cell membranes contain a fucosylated form of iGb3. Neutral glycosphingolipids membranes from WT and GalT KO PAEC were extracted, permethylated, and analyzed by MS. Molecular ion profiles of neutral glycolipid membrane fractions of WT (A) and GalT KO (B) PAEC obtained by linear ion trap mass spectrometer using the electrospray ionization mass spectroscopic method (LTQ-ESI-MS) are shown. The presence of fucosylated iGb3 structure was found by precursor ion mapping method (using 841 as the product ion). Candidate structures for the ion are Galα1,3(Fucα1,2) Galβ1,4Glc-Cer, and/or Fucα1,2Galα1,3Galβ1,4Glc-Cer. Spectra x-axe in m/z. Cer, ceramide; GalT KO, α1,3galactosyltransferase knockout; Gb3, globotrihexosylceramide 3, iGb3, isoglobotrihexo sylceramide; MS, mass spectrometry; PAEC, pig aortic endothelial cells; WT, wild-type.
α1,3galactosyltransferase activity in pig endothelial cell lysates
Hitherto, analysis of the GalT enzymatic activity in cells derived from GalT KO pigs has not been reported. Theoretically, other enzymes originally unable to compete against GalT for UDP-Gal in WT cells could be active in GalT KO cells. Thus, we analyzed the enzymatic activity in freshly prepared cell lysates from WT and GalT KO PAEC by measuring the transfer of 14C-labeled UDP-Gal residues into the acceptor molecule asialofetuin. As shown in Table 1, a minimum (0.0018 mU/g), if not null, enzymatic activity was found in GalT KO PAEC lysates after 1 h, and this activity stayed the same during the 3 h of observation. On the contrary, WT PAEC clearly produced a labeled adduct at 1 h (0.0263 mU/g), which increased over time (0.0364 mU/g). Used as positive control, human E293 cells transfected with rat GalT, also showed an increase in αGal-asialofetuin formation over time, although the enzymatic activity detected was lower (0.0031 and 0.0061 mU/g at 1 and 3 h, respectively) than in WT PAEC lysates. Finally, 0.7 μg of commercially available purified human βGal1,4GalT with an enzymatic activity of 250 pmol/min/μg was tested under the same conditions to analyze whether βGal1,4GalT enzymatic activity could account for the results obtained in the above-mentioned assays. We detected 1.03 nmol of UDP-Gal/1 h under saturating conditions, while the expected activity of β1,4GalT was nearly 10 times higher (10.5 nmol/h/0.7 μg). In conclusion, GalT enzymatic activity was detected in WT PAEC and not in GalT KO PAEC.
Table 1.
α1,3galactosyltransferase activity detected in cell lysates
| 1 h
|
3 h
|
|||
|---|---|---|---|---|
| nmol/60 min/150 μg | mU/g | nmol/180 min/150 μg | mU/g | |
| PAEC WT | 0.243 | 0.0263 | 0.3278 | 0.0364 |
| PAEC GalT KO | 0.014 | 0.0018 | 0.0154 | 0.0017 |
| E293 rat GalT | 0.032 | 0.0031 | 0.0546 | 0.0061 |
GalT, α1,3galactosyltransferase; KO, knock-out; PAEC, pig aortic endothelial cells; WT, wild-type.
One hundred and fifty micrograms of cell lysates from pig aortic endothelial cells from WT and GalT KO animals were incubated in the presence of UDP14[C]-Gal and asialofetuin at 37 °C. Enzymatic reactions were stopped after 1 and 3 h and radioactivity incorporation was quantified. As positive control, human kidney embryonic cells (E293) transfected with rat GalT were used.
Levels of isoglobotrihexosylceramide synthase mRNA in WT and GalT KO pigs
As we could not find iGb3 in WT and GalT KO PAEC, we wondered whether the enzyme that catalyzes the production of iGb3 is actually expressed in pig cells. To this purpose, the mRNA levels of iGb3S were analyzed not only in PAEC but also in different tissues derived from WT and GalT KO animals. We used three different sets of primers to detect iGb3S: K2/K3, K2/K4, and 67/68, respectively (Fig. 5A). The products were inserted into the cloning site of the pGEM-T expression vector and sequenced. Whereas the K2/K4 and 67/68 amplified products presented homology to a predicted pig alpha-centractin and other DNA sequences present in pig chromosomes 4, 7, 17, and 18, respectively, only the K2/K3 amplified product matched the known sequence of Sus scrofa iGb3S (patent WO 2005/047469). Due to the fact that the K2/K3 primer set spans exclusively exon 5 of the iGb3S gene, negative controls without reverse transcriptase were always included to rule out genomic DNA contaminations. Both WT and GalT KO PAEC expressed iGb3S mRNA. In accordance, all pig tissues tested expressed considerable amounts of mRNA for exon-5 iGb3S regardless of their anatomical origin or their GalT status (Fig. 5B). Of note, kidney tissue showed the highest levels of iGb3S mRNA, whereas liver tissue exhibited clearly lower levels of expression. No apparent differences were found between thymus and spleen iGb3S mRNA expression. Although these results are not quantitative, β2 microglobulin mRNA was equivalent among all samples tested. In summary, iGb3S mRNA was broadly expressed in all pig tissues tested whether derived from WT or GalT KO animals.
Fig. 5.
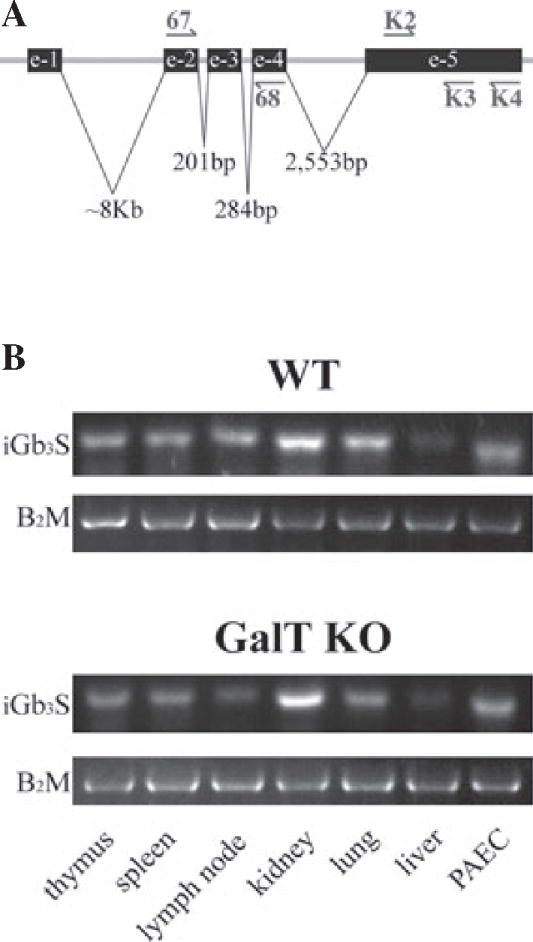
Expression of mRNA levels of the isoglobotrihexosylceramide 3 synthase in pig aortic endothelial cells and pig tissues. Different tissues were isolated from just sacrificed animals and snap frozen for RNA extraction and analysis. The mRNA levels for iGb3S were checked with three different set of primers. Location of primers is shown using the patented WO 2005/047469 sequence as reference (A). Pig β2-microglobulin was used as a housekeeping gene. RT-PCR products of K2/K3 primers set were run in 2% agarose gel electrophoresis (B). Two different samples from each organ were obtained from a WT animal (#16517, female, AA haplotype, 2.5 yr old) and a GalT KO animal (#16183, female, DD haplotype, 3.5 yr old), respectively. e, exon; GalT KO, α1,3galactosyltransferase knockout; iGb3S, isoglobotrihexosylceramide 3 synthase; mRNA, messenger ribonucleic acid; PAEC, pig aortic - endothelial cells.
Discussion
The discovery of iGb3S that is able to form terminal Galα1,3Gal disaccharides on lipids has been a source of discussion regarding the theoretical alternative expression of αGal epitopes on GalT KO pig cells [10]. This work simultaneously tested all available Ab anti-αGal including polyclonal human Ab, seven different mAb anti-αGal, and two lectins as well as highly sensitive MS techniques to confirm that αGal is not present in GalT KO PAEC, at least not in GalT KO pigs generated by Immerge [11]. Moreover, we report GalT enzymatic activity and iGb3S gene transcription in WT and GalT KO cells and tissues.
None of the above-mentioned reagents used to determine αGal expression provided positive staining of GalT KO PAEC using flow cytometry and immunofluorescence microscopy, strongly suggesting that at least at this level of detection, there is no αGal expression on the surface of the Immerge GalT KO cells. Noteworthy, flow cytometry of WT PAEC using the 15.101 mAb yielded positive staining in our hands, whereas GalT KO PAEC were not stained by 15.101. This antibody was initially reported to be specific for iGb3 using human embryonic kidney cells (E293) transfected with rat iGb3S, and Chinese hamster ovary cells (CHO) transfected with mouse iGb3S [8,13]. However, subsequently, the 15.101 mAb was demonstrated by Diswall et al. [25] to cross-react to Gb3 and to stain porcine WT intestinal cell extracts in thin-layer chromatography plates while GalT KO tissue remained negative. Taken together, these results suggest that controversial reports regarding the specificity of the 15.101 mAb might depend on discrepancies of the techniques used to demonstrate binding and that the 15.101 mAb might also bind to αGal. Hence, the data obtained with this mAb should be interpreted very carefully.
With regard to the other mAb tested, we did not have access to the mAb GT-21-1-G1.6 and GT-21-1-G1.8 that were reported to stain positive for αGal expression on pig GalT KO fibroblasts by Sharma et al. [26]. The latter mAb (GT-21-1-G1.6 and GT-21-1-G1.8) were subsequently shown to have a lower affinity for the αGal disaccharide in comparison with other IgG mAb developed by the same group, necessitating at least eight disaccharides conjugated to proteins for recognition [27]. However, we obtained the mAb GT4-31 and GT6-27 described in the same study, which both stained exclusively WT PAEC. Thus, to finally reconcile the discrepancy of the specificity of these mAb, we suggest them to be assayed by the “Consortium of Functional Glycomics” (http://www.functionalglycomics.org) to clarify whether they specifically recognize the αGal epitope or also other saccharide chains. In conclusion, it is important to mention that if there is no detectable antibody binding to pig-derived cells/tissues, the risk of antibody-mediated rejection in pig-to-primate xenotransplantation is expected to be negligible.
The glycosphingolipid iGb3 has been described as a possible source of the αGal epitope in mice and rats [8,9]. It is synthesized by the enzyme iGb3S using LacCer as precursor [28,29] and characterized by the presence of the αGal in the terminal part [10]. In the second part of our study, we attempted to demonstrate iGb3 expression in WT and GalT KO PAEC using sophisticated MS techniques. The MS analysis was performed on the neutral membrane extracts of GalT KO PAEC because it is in this fraction where iGb3 can be found and not in the acidic fractions or membrane proteins as reported by others [30,31]. Nevertheless, the highly sensitive ion trap MS analysis identified the presence of Gb3 at a fmol level [18] and confirmed the lack of the αGal epitope in the lipidic fraction of GalT KO PAEC. Furthermore, iGb3 was neither detected in WT nor GalT KO PAEC using as much as 100 million cells for the analysis, despite the presence of its precursor LacCer. Similar findings were reported by Diswall et al. [32] analyzing heart and kidney material stemming from Immerge’s GalT KO pigs by MS and proton nuclear magnetic resonance (NMR) where neither terminal αGal nor iGb3 were found in the neutral lipidic fractions. In our analysis of the neutral glycosphingolipids membrane fractions, we detected αGal in the form of Galα1,3Galβ1,4GlcβNAcβ1,3-LacCer in WT, but not in GalT KO PAEC. Similar conclusions were reported after analyzing lipidic fractions of small intestine and pancreas of GalT KO Revivicor animals using thin-layer chromatography and proton NMR by Diswall et al. [33,34]. In conclusion, if there is any iGb3 expression in WT and GalT KO PAEC, it escapes detection using highly sensitive methods and should, therefore, not be relevant for transplantation.
Regarding the iGb3-fucosylated structure identified in both cells types, we do not know whether the fucose is terminal or not. From the original work in pig stomach mucosa [35] and later findings of Diswall et al. [33], we presume that Fuc-iGb3 most likely corresponds to the glycolipid Fucα1,2Galα1,3Galβ1,4Glcβ1,1-Cer, which was also found in both WT and GalT KO pig intestine [33,35]. The fact that the 4F10 mAb does not recognize the Galα1,3(Fuc α1,2)Galβ1,4Glc glycans (Fig. S1) did not help us to directly define the precise position of the fucose, because the level of Fuc-iGb3 present in PAEC is under the detection limit for mAb determinations. On the other hand, the recognition of terminal fucosylated structures in PAEC by UEA-I lectin cannot be explained by the low abundance of the Fuc-iGb3 (MS signal of 1389 m/z). The increased blood group H-type structures revealed by UEA-I staining in GalT KO PAEC has previously been reported and is possibly related to the unmasking of fucose epitopes in the absence of GalT [36]. An alternative pathway leading to a branched fucose epitope in which the fucose is attached to the internal galactose (Galα1,3(Fucα1,2)Galβ1,3Glc-Cer) is independent of the iGb3 pathway. In this case, LacCer fucosylated by a fucosyltransferase I, a structure that was found in low abundance in WTPAEC membranes (Fig. S3) by MS, might serve as a substrate for additional transferases. In conclusion, the structural characterization of the fucosylated iGb3 structure which we observed was incomplete, and we can only speculate that it might be synthesized by different fucosyltransferase enzymes such as Fut7 (EC 2.4.1.65) or Fut1 and/or Fut2 (EC 2.4.1.69).
Another point we addressed was the GalT enzymatic activity of PAEC. We used an enzymatic assay based on the incorporation of radioactive UDP-Gal residues into the asialofetuin acceptor substrate [20–22]. GalT acts on βgalactosyl1,4 N-acetylglucosaminyl termini, asialo- α1-acid glycoprotein, N-acetyllactosamine, and non-reducing terminal N-acetyllactosamine residues of glycoproteins but not on 2′-fucosylated-N-acetyllactosamine [37]. Our results clearly show that GalT activity is not present in cell lysates from GalT KO PAEC when asialofetuin was used as a substrate. As asialofetuin bears the terminal galactose (Gal β4GlcNAc) in N-glycans, we were assaying the α1,3GalT activity toward the terminal galactose. We think that the low and constant activity registered in PAEC WT is due to background because in the case of E293 transfected with rat GalT, the enzymatic activity doubled after 3 h, which is not the case for PAEC WT.
Finally, although we could not detect iGb3 in the glycolipid neutral fractions by spectrometry, iGb3S mRNA was present in all pig tissues and cells tested, regardless whether derived from WT or GalT KO animals. The possibility that iGb3 was further converted into other elongated isoglobo-series glycosphingolipids, such as iGb4, iGb5 and iGb6 glycosphingolipids [38], was excluded by mass spectrometry (data not shown). The mechanism responsible for the absence of the iGb3S protein in the presence of its mRNA remains to be clarified. It is possible that the iGb3S lacks a cofactor for enzymatic activity or that there are genetic differences between the source animals. Unpublished data from Christiansen et al. (M. Sandrin, personal communication) support the latter notion as they found differences in iGb3S exon 5 sequences in different pig sources, revealing a stop codon in exon 5 of iGb3S in Immerge’s breed animals. Importantly, the primer set K2/K3 that proved iGb3S mRNA expression is located before this stop codon. The discrepancy between our results and Kiernan et al. who did not detect iGb3S by RT-PCR testing the same animal source material [23] might stem from the different amplification conditions used, in particular the annealing temperature that differed by 7 °C. Nevertheless, the sequence of our amplification products obtained with the K2/K3 primer pair matched the pig iGb3S gene.
In summary, this work confirms previously published evidence and concludes the earlier controversy about the expression of the terminal αGal disaccharide on the cell surface of GalT KO animals. Neither αGal nor iGb3 were detected in Immerge’s GalT KO breed animals using a panel of different antibodies and a highly sensitive MS method. Most importantly, the lack of αGal antigen recognition by polyclonal human anti-Gal antibodies on GalT KO cells, not even at trace levels, indicates that antibody-mediated delayed xenograft rejection is not related to residual αGal expression. Thus, the xenoantigens responsible for delayed xenograft rejection need to be identified and addressed by novel preventive strategies [7,23,39–44].
Supplementary Material
Acknowledgments
The authors want to thank David Sachs and John Hanekamp (Massachusetts General Hospital, Boston, MA, USA) for providing pig tissues; Dale Christiansen for critical reading of the manuscript; Prof. Hennet (University of Zurich, Institute of Pathology, Zurich, Switzerland) for providing UDP-[U-14C]Gal; Mauro Sandrin is acknowledged for providing reagents, helpful discussions, and critical reading of the manuscript; and the Glyco-Chip array by Consortium of Functional Glycomics at the Scripps Research Institute, USA for the specificity test of 4F10 mAb.
Funding
This work was supported by the Krebsliga des Kantons Zürich; UBS AG on behalf of a client; the Swiss National Science Foundation [3200B0-109921; 3100A0-116295; 320030-138376]; and contributions of the Wilsdorf Foundation; Christian Loepfe in support of transplantation research, Switzerland; the University of Texas MD Anderson Center at Houston. DZ is supported by MD Anderson Cancer Center and NIH grants AI079232, AI078898, a developmental award and a supplemental award from P30-AI36211.
Abbreviations
- αGal
Galα1,3Gal disaccharide
- Fuc
fucose
- Gal
galactose
- GalT
α1,3galactosyltransferase
- Gb3
globotrihexosylceramide 3 (Galα1,4-Galβ1,4Glcβ1-ceramide)
- iGb3
isoglobotrihexosylceramide 3 (Galα1,3- Galβ1,4Glcβ1-ceramide)
- iGb3S
isoglobotrihexosylceramide 3 synthase
- LacCer
lactosylceramide (Galβ1,4Glcβ1-ceramide)
- LTQ-ESI-MS
linear ion trap electrospray ionization mass spectroscopy
- mAb
monoclonal antibody
- MFIR
mean fluorescence intensity ratio
- MS
mass spectroscopy
- PAEC
pig aortic endothelial cells
- RFU
relative fluorescence unit
Footnotes
Authors’ contributions
GPY, JDS contributed to the concept/design of the article; GPY, YL, LB, ALM, MBK, DZ performed data analysis/interpretation; GPY drafted the article; and DZ, LB, ALM, JDS were involved in the critical revision of the article.
Conflict of interest
DZ is a consultant for BioTex, Houston, TX, and an inventor involved in patents related to technologies mentioned in this article, issued or in application.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Specificity of 4F10 monoclonal antibody determined by the Glycan-array assay.
Figure S2. Tandem Mass Spectrometry detects iGb3 from isomeric mixtures after multiple rounds of fragmentation that leads to characteristic ions.
Figure S3. Precursor of fucosylated lactosylceramide in pig aortic endothelial cell membranes
Table S1. Structures recognized by the different antibodies and lectins used in the study.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Tai HC, Zhu X, Hara H, et al. The pig-to-primate immune response: relevance for xenotransplantation. Xenotransplantation. 2007;14:227–235. doi: 10.1111/j.1399-3089.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Waer M, Billiau AD. Xenotransplantation: role of natural immunity. Transpl Immunol. 2009;21:70–74. doi: 10.1016/j.trim.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Macher BA, Galili U. The Galalpha1,3Galbeta1,4Glc-NAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta. 2008;1780:75–88. doi: 10.1016/j.bbagen.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 5.Hisashi Y, Yamada K, Kuwaki K, et al. Rejection of cardiac xenografts transplanted from a 1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am J Transplant. 2008;8:2516–2526. doi: 10.1111/j.1600-6143.2008.02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 7.Puga YG, Schneider MK, Seebach JD. Immune responses to alpha1,3 galactosyltransferase knockout pigs. Curr Opin Organ Transplant. 2009;14:154–160. doi: 10.1097/MOT.0b013e328329250d. [DOI] [PubMed] [Google Scholar]
- 8.Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol. 2006;176:2448–2454. doi: 10.4049/jimmunol.176.4.2448. [DOI] [PubMed] [Google Scholar]
- 9.Taylor SG, Mckenzie IF, Sandrin MS. Characterization of the rat alpha(1,3)galactosyltransferase: evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1,3)Gal by two separate glycosylation pathways. Glycobiology. 2003;13:327–337. doi: 10.1093/glycob/cwg030. [DOI] [PubMed] [Google Scholar]
- 10.Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU. Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J Biol Chem. 2000;275:25308–25314. doi: 10.1074/jbc.M002629200. [DOI] [PubMed] [Google Scholar]
- 11.Kolber-Simonds D, Lai L, Watt SR, et al. Production of alpha-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci USA. 2004;101:7335–7340. doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann BC, Schneider MK, Lilienfeld BG, et al. Endothelial cells derived from pigs lacking Gal alpha(1,3)Gal: no reduction of human leukocyte adhesion and natural killer cell cytotoxicity. Transplantation. 2005;79:1067–1072. doi: 10.1097/01.tp.0000157231.11083.7c. [DOI] [PubMed] [Google Scholar]
- 13.Milland J, Yuriev E, Xing PX, Mckenzie IF, Ramsland PA, Sandrin MS. Carbohydrate residues down-stream of the terminal Galalpha(1,3)Gal epitope modulate the specificity of xenoreactive antibodies. Immunol Cell Biol. 2007;85:623–632. doi: 10.1038/sj.icb.7100111. [DOI] [PubMed] [Google Scholar]
- 14.Baumann BC, Forte P, Hawley RJ, Rieben R, Schneider MK, Seebach JD. Lack of galactose-alpha-1,3-galactose expression on porcine endothelial cells prevents complement-induced lysis but not direct xenogeneic NK cytotoxicity. J Immunol. 2004;172:6460–6467. doi: 10.4049/jimmunol.172.10.6460. [DOI] [PubMed] [Google Scholar]
- 15.Nozawa S, Xing PX, Wu GD, et al. Characteristics of immunoglobulin gene usage of the xenoantibody binding to gal-alpha(1,3)gal target antigens in the gal knockout mouse. Transplantation. 2001;72:147–155. doi: 10.1097/00007890-200107150-00028. [DOI] [PubMed] [Google Scholar]
- 16.Yin D, Zeng H, Ma L, et al. Cutting Edge: NK cells mediate IgG1-dependent hyperacute rejection of xenografts. J Immunol. 2004;172:7235–7238. doi: 10.4049/jimmunol.172.12.7235. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zhou D, Xia C, Wang PG, Levery SB. Sensitive quantitation of isoglobotriaosylceramide in the presence of isobaric components using electrospray ionization-ion trap mass spectrometry. Glycobiology. 2008;18:166–176. doi: 10.1093/glycob/cwm127. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Teneberg S, Thapa P, Bendelac A, Levery SB, Zhou D. Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology. 2008;18:158–165. doi: 10.1093/glycob/cwm129. [DOI] [PubMed] [Google Scholar]
- 19.Malissard M, Borsig L, Di Marco S, et al. Recombinant soluble beta-1,4-galactosyltransferases expressed in Saccharomyces cerevisiae. Purification, characterization and comparison with human enzyme. Eur J Biochem. 1996;239:340–348. doi: 10.1111/j.1432-1033.1996.0340u.x. [DOI] [PubMed] [Google Scholar]
- 20.Borsig L, Katopodis AG, Bowen BR, Berger EG. Trafficking and localization studies of recombinant alpha1, 3-fucosyltransferase VI stably expressed in CHO cells. Glycobiology. 1998;8:259–268. doi: 10.1093/glycob/8.3.259. [DOI] [PubMed] [Google Scholar]
- 21.Latemple DC, Henion TR, Anaraki F, Galili U. Synthesis of alpha-galactosyl epitopes by recombinant alpha1,3galactosyl transferase for opsonization of human tumor cell vaccines by anti-galactose. Cancer Res. 1996;56:3069–3074. [PubMed] [Google Scholar]
- 22.Leventhal JR, Sun J, Zhang J, et al. Evidence that tilapia islets do not express alpha-(1,3)gal: implications for islet xenotransplantation. Xenotransplantation. 2004;11:276–283. doi: 10.1111/j.1399-3089.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiernan K, Harnden I, Gunthart M, Gregory C, Meisner J, Kearns-Jonker M. The anti-non-gal xenoantibody response to xenoantigens on gal knockout pig cells is encoded by a restricted number of germline progenitors. Am J Transplant. 2008;8:1829–1839. doi: 10.1111/j.1600-6143.2008.02337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christiansen D, Milland J, Mouhtouris E, et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008;6:e172. doi: 10.1371/journal.pbio.0060172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diswall M, Gustafsson A, Holgersson J, Sandrin MS, Breimer ME. Antigen-binding specificity of anti-alphaGal reagents determined by solid-phase glycolipid-binding assays. A complete lack of alphaGal glycolipid reactivity in alpha1,3GalT-KO pig small intestine. Xenotransplantation. 2011;18:28–39. doi: 10.1111/j.1399-3089.2011.00623.x. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Naziruddin B, Cui C, et al. Pig cells that lack the gene for alpha1-3 galactosyltransferase express low levels of the gal antigen. Transplantation. 2003;75:430–436. doi: 10.1097/01.TP.0000053615.98201.77. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Yin D, Naziruddin B, et al. The in vitro and in vivo effects of anti-galactose antibodies on endothelial cell activation and xenograft rejection. J Immunol. 2003;170:1531–1539. doi: 10.4049/jimmunol.170.3.1531. [DOI] [PubMed] [Google Scholar]
- 28.Heissigerova H, Breton C, Moravcova J, Imberty A. Molecular modeling of glycosyltransferases involved in the biosynthesis of blood group A, blood group B, Forssman, and iGb3 antigens and their interaction with substrates. Glycobiology. 2003;13:377–386. doi: 10.1093/glycob/cwg042. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Thapa P, Hawke D, et al. Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J Proteome Res. 2009;8:2740–2751. doi: 10.1021/pr801040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YG, Gil GC, Harvey DJ, Kim BG. Structural analysis of alpha-Gal and new non-Gal carbohydrate epitopes from specific pathogen-free miniature pig kidney. Proteomics. 2008;8:2596–2610. doi: 10.1002/pmic.200700972. [DOI] [PubMed] [Google Scholar]
- 31.Kim YG, Gil GC, Jang KS, et al. Qualitative and quantitative comparison of N-glycans between pig endothelial and islet cells by high-performance liquid chromatography and mass spectrometry-based strategy. J Mass Spectrom. 2009;44:1087–1104. doi: 10.1002/jms.1587. [DOI] [PubMed] [Google Scholar]
- 32.Diswall M, Angstrom J, Karlsson H, et al. Structural characterization of alpha1,3-galactosyltransferase knockout pig heart and kidney glycolipids and their reactivity with human and baboon antibodies. Xenotransplantation. 2010;17:48–60. doi: 10.1111/j.1399-3089.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- 33.Diswall M, Angstrom J, Schuurman HJ, Dor FJ, Rydberg L, Breimer ME. Studies on glycolipid antigens in small intestine and pancreas from alpha1,3-galactosyltransferase knockout miniature swine. Transplantation. 2007;84:1348–1356. doi: 10.1097/01.tp.0000287599.46165.15. [DOI] [PubMed] [Google Scholar]
- 34.Diswall M, Angstrom J, Schuurman HJ, Dor FJ, Rydberg L, Breimer ME. Glycolipid studies in small intestine and pancreas of alpha1,3-galactosyltransferase knockout miniature swine: alpha1,3GALT-KO animals lack alphaGAL antigens and contain novel blood group H compounds. Transplant Proc. 2008;40:543–546. doi: 10.1016/j.transproceed.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 35.Slomiany BL, Slomiany A, Horowitz MI. Characterization of blood-group-H-active ceramide tetrasaccharide from hog-stomach mucosa. Eur J Biochem. 1974;43:161–165. doi: 10.1111/j.1432-1033.1974.tb03396.x. [DOI] [PubMed] [Google Scholar]
- 36.Hara H, Long C, Lin YJ, et al. In vitro investigation of pig cells for resistance to human antibody-mediated rejection. Transpl Int. 2008;21:1163–1174. doi: 10.1111/j.1432-2277.2008.00736.x. [DOI] [PubMed] [Google Scholar]
- 37.Scheer M, Grote A, Chang A, et al. BRENDA, the enzyme information system in 2011. Nucleic Acids Res. 2011;39(Database issue):D670–D676. doi: 10.1093/nar/gkq1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou D, Levery SB. Response to Milland et al: carbohydrate residues downstream of the terminal Galalpha( 1,3)Gal epitope modulate the specificity of xenoreactive antibodies. Immunol Cell Biol. 2008;86:631–632. doi: 10.1038/icb.2008.65. author reply 633–634. [DOI] [PubMed] [Google Scholar]
- 39.Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 2007;83:193–201. doi: 10.1097/01.tp.0000250478.00567.e5. [DOI] [PubMed] [Google Scholar]
- 40.Byrne GW, Stalboerger PG, Davila E, et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation. 2008;15:268–276. doi: 10.1111/j.1399-3089.2008.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obukhova P, Rieben R, Bovin N. Normal human serum contains high levels of anti-Gal alpha 1-4GlcNAc antibodies. Xenotransplantation. 2007;14:627–635. doi: 10.1111/j.1399-3089.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 42.Pierson RN, III, Dorling A, Ayares D, et al. Current status of xenotransplantation and prospects for clinical application. Xenotransplantation. 2009;16:263–280. doi: 10.1111/j.1399-3089.2009.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rood PP, Tai HC, Hara H, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20:1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 44.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.