Table 2.
Substrate scope: α-imino ester.
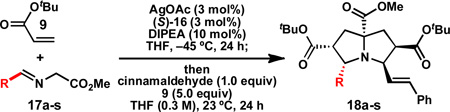 | |||||||
|---|---|---|---|---|---|---|---|
| entry | yielda (%) |
eeb (%) |
entry | yielda (%) |
eeb (%) |
||
| 1 | 90 | 91 | 11d | 86 | 90 | ||
| 2 | 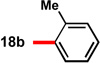 |
91 | 91 | 12 | 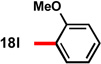 |
72 | 90 |
| 3c | 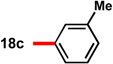 |
83 | 88 | 13 | 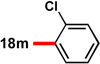 |
87 | 94 |
| 4c | 82 | 92 | 14 | 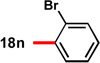 |
89 | 93 | |
| 5c | 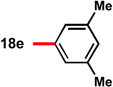 |
78 | 88 | 15 | 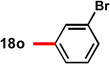 |
82 | 92 |
| 6 | 87 | 93 | 15d | 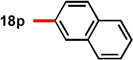 |
76 | 92 | |
| 7 | 91 | 95 | 17c | 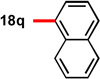 |
84 | 93 | |
| 8 | 89 | 92 | 18 | 90 | 90 | ||
| 9 | 70 | 96 | |||||
| 10 | 80 | 96 | 19 | 33 | 44 | ||
Isolated yield.
Determined by SFC using chiral stationary phase.
6 mol % each AgOAc and (S)-16 were employed.
6 mol % each AgOAc and (S)-16 were employed at 0.1 M reaction concentration.
