Figure 3. Unique physical properties of the mammalian CENP-A-containing nucleosome.
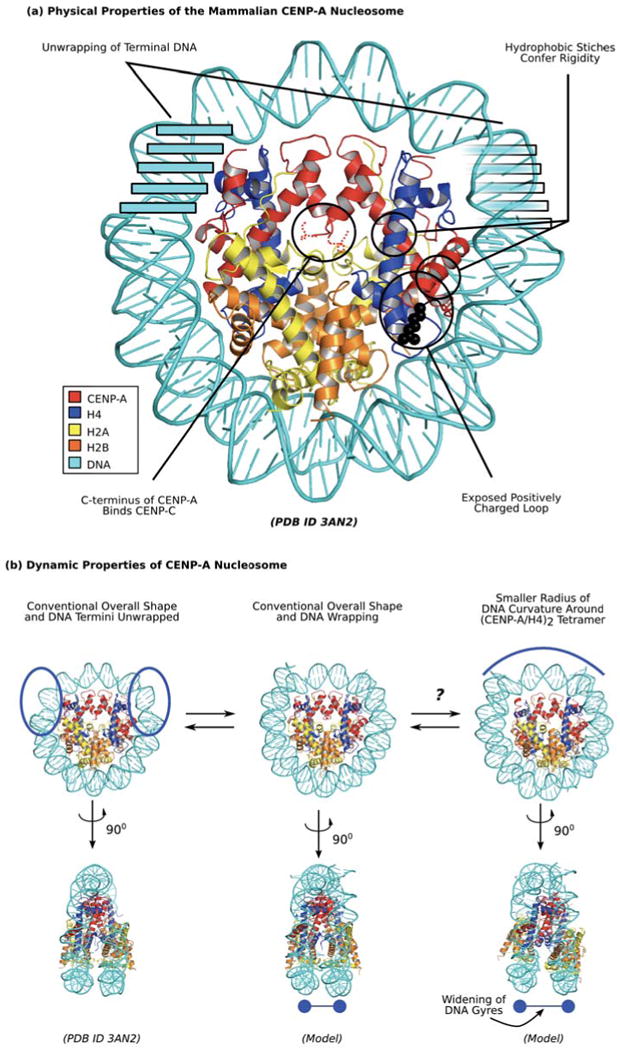
(a) Distinguished physical properties of CENP-A nucleosome are highlighted in black circles. Clockwise from the top left, these include transient unwrapping/flexibility of the final helical turn of DNA at each nucleosome terminus [14,57,58], hydrophobic stitches that rigidify the CENP-A/H4 interface [13,50,54,56], a bulged loop L1 that is of opposite charge as on H3 in the conventional nucleosome [13,14], and the unstructured C-terminus that mediates recognition of CENP-A nucleosomes by CENP-C [83]. (b) Dynamic properties of CENP-A nucleosome indicate potential sampling between distinct conformations. Mammalian CENP-A containing nucleosomes wrap ∼145 bp of DNA at steady-state (center; [13,58]), but sample unwrapping at the terminal DNA to a greater extent than conventional nucleosomes with canonical H3 [57,58]. The crystal form of the nucleosome includes only 120 bp of DNA that is visible in the structure (left; [14]), supporting the notion of increased flexibility at nucleosome termini. Another potential alternate form under investigation is one where rotation at the CENP-A/CENP-A is maintained as in the isolated sub-nucleosomal (CENP-A/H4)2 heterotetramer [13], leading to a smaller radius of curvature of DNA wrapping near the nucleosomal dyad (right, top) and wider spacing of H2A/H2B heterodimers and the two strands of nucleosomal DNA (right, bottom).
