Abstract
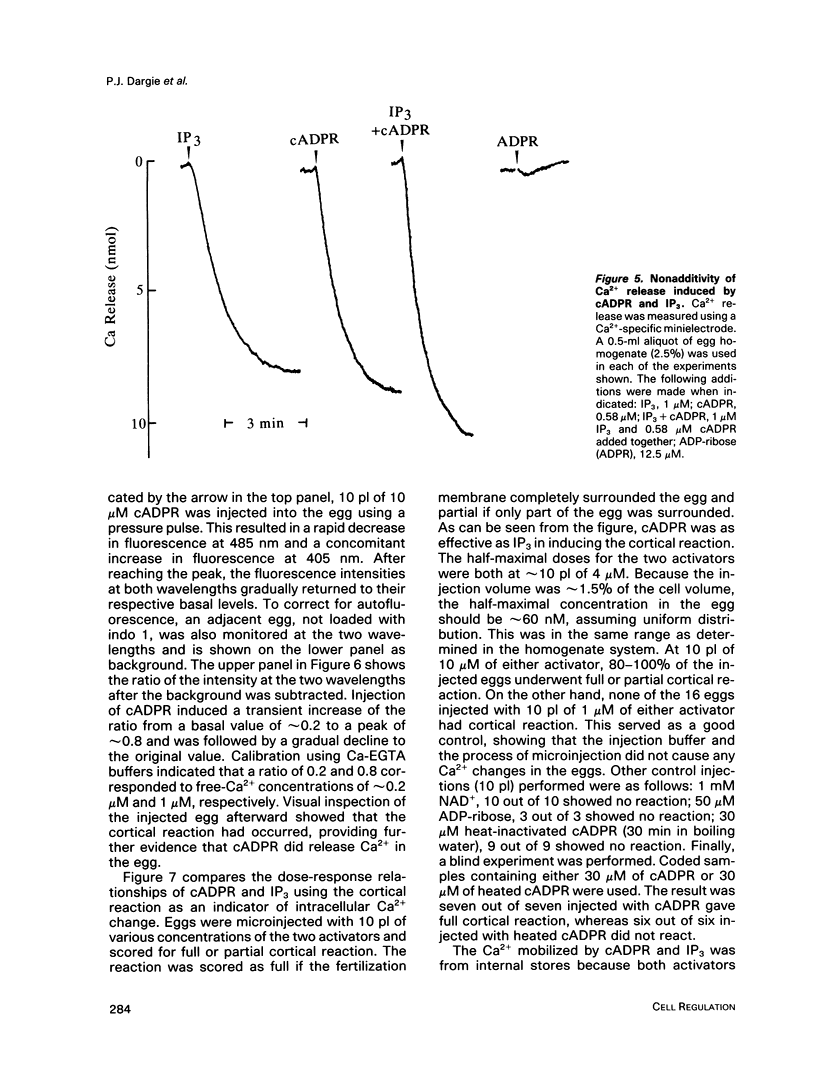
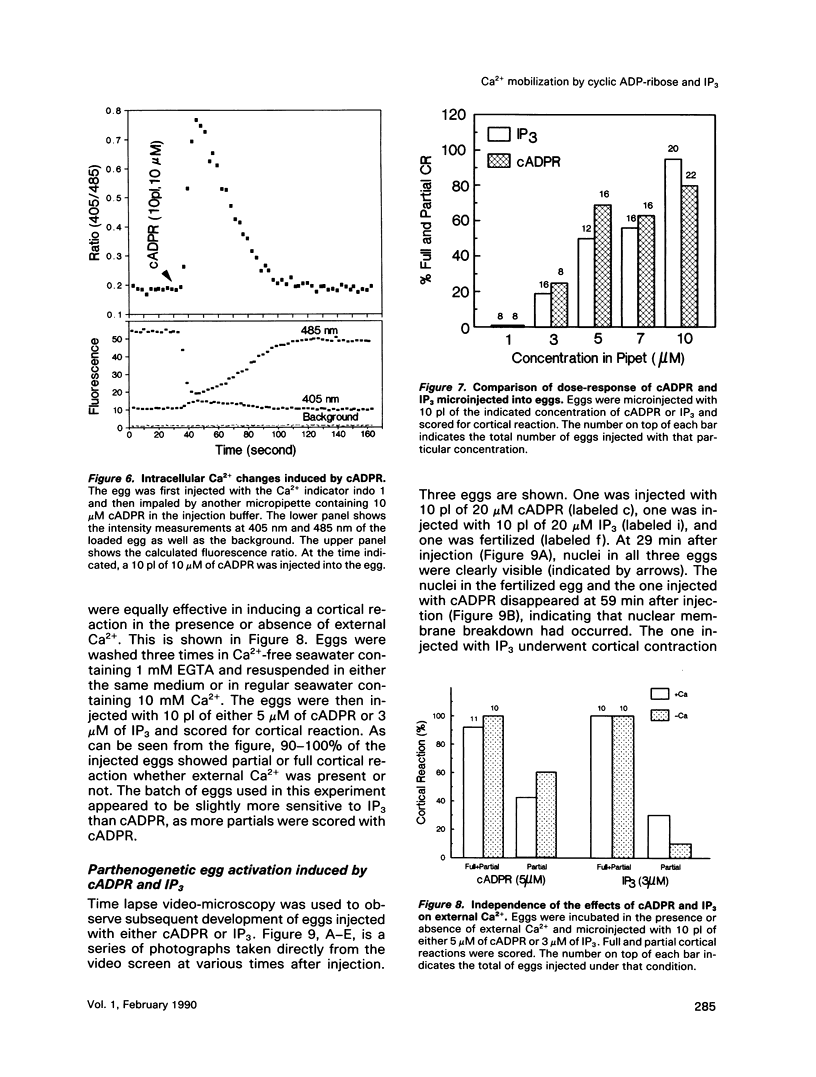
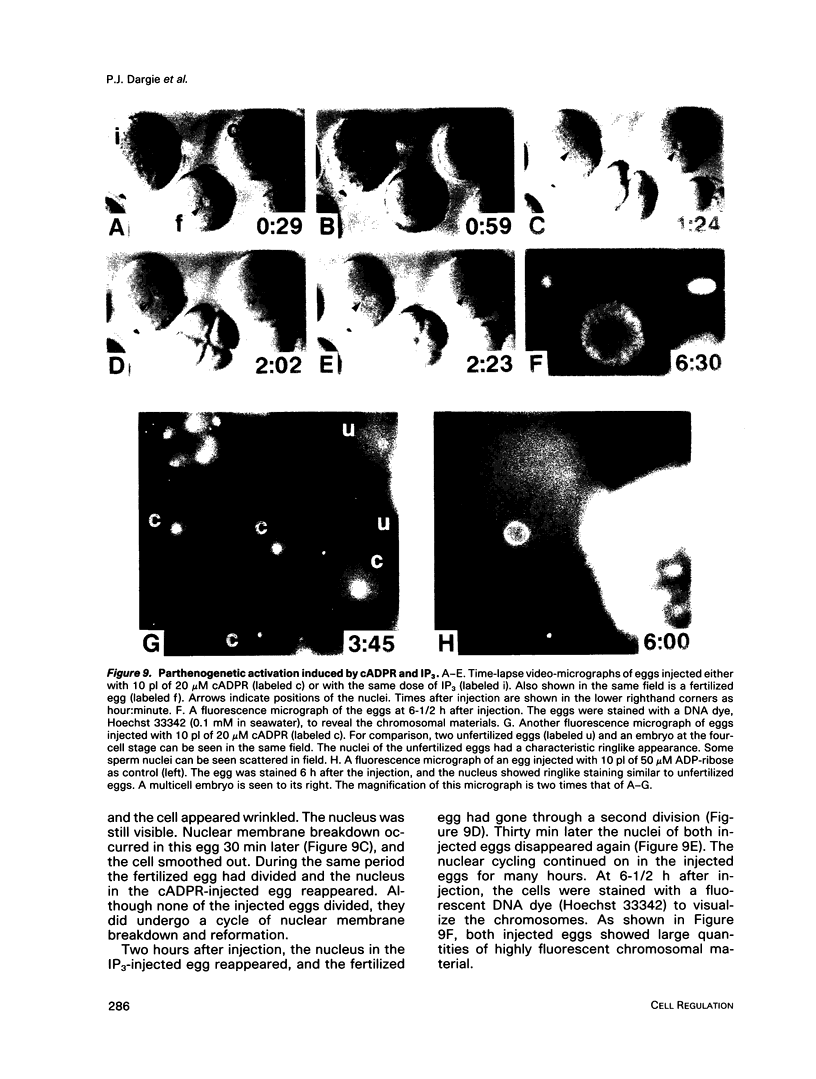
We have previously shown that a metabolite of NAD+ generated by an enzyme present in sea urchin eggs and mammalian tissues can mobilize intracellular Ca2+ in the eggs. Structural determination established it to be a cyclized ADP-ribose, and the name cyclic ADP-ribose (cADPR) has been proposed. In this study, Ca2+ mobilizations induced by cADPR and inositol trisphosphate (IP3) in sea urchin egg homogenates were monitored with Ca2+ indicators and Ca2(+)-specific electrodes. Both methods showed that cADPR can release Ca2+ from egg homogenates. Evidence indicated that it did not act as a nonspecific Ca2(+)-ionophore or as a blocker of the microsomal Ca2(+)-transport; instead, it was likely to be operating through a specific receptor system. This was supported by its half-maximal effective concentration of 18 nM, which was 7 times lower than that of IP3. The receptor for cADPR appeared to be different from that of IP3 because heparin, an inhibitor of IP3 binding, had no effect on the cADPR action. The Ca2+ releases induced by cADPR and IP3 were not additive and had an inverse relationship, indicating overlapping stores were mobilized. Microinjection of cADPR into intact eggs induced transient intracellular Ca2+ changes and activated the cortical reaction. The in vivo effectiveness of cADPR was directly comparable with IP3 and neither required external Ca2+. In addition, both were effective in activating the eggs to undergo multiple nuclear cycles and DNA synthesis. These results suggest that cADPR could function as a second messenger in sea urchin eggs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Changya L., Gallacher D. V., Irvine R. F., Potter B. V., Petersen O. H. Inositol 1,3,4,5-tetrakisphosphate is essential for sustained activation of the Ca2+-dependent K+ current in single internally perfused mouse lacrimal acinar cells. J Membr Biol. 1989 Jul;109(1):85–93. doi: 10.1007/BF01870793. [DOI] [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J Biol Chem. 1987 Jul 15;262(20):9561–9568. [PubMed] [Google Scholar]
- Eisen A., Kiehart D. P., Wieland S. J., Reynolds G. T. Temporal sequence and spatial distribution of early events of fertilization in single sea urchin eggs. J Cell Biol. 1984 Nov;99(5):1647–1654. doi: 10.1083/jcb.99.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H. H., Derr J. E. Assay of inorganic and organic phosphorus in the 0.1-5 nanomole range. Anal Biochem. 1975 Feb;63(2):607–613. doi: 10.1016/0003-2697(75)90388-7. [DOI] [PubMed] [Google Scholar]
- Kamel L. C., Bailey J., Schoenbaum L., Kinsey W. Phosphatidylinositol metabolism during fertilization in the sea urchin egg. Lipids. 1985 Jun;20(6):350–356. doi: 10.1007/BF02534201. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jan 25;264(3):1608–1615. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Rusinko N., Lee H. C. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J Biol Chem. 1989 Jul 15;264(20):11725–11731. [PubMed] [Google Scholar]
- Sawyer D. W., Sullivan J. A., Mandell G. L. Intracellular free calcium localization in neutrophils during phagocytosis. Science. 1985 Nov 8;230(4726):663–666. doi: 10.1126/science.4048951. [DOI] [PubMed] [Google Scholar]
- Schmidt T., Patton C., Epel D. Is there a role for the Ca2+ influx during fertilization of the sea urchin egg? Dev Biol. 1982 Apr;90(2):284–290. doi: 10.1016/0012-1606(82)90377-3. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Swann K., Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986 Dec;103(6 Pt 1):2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Connolly T. M., Bross T. E., Majerus P. W., Sherman W. R., Tyler A. N., Rubin L. J., Brown J. E. Isolation and characterization of the inositol cyclic phosphate products of polyphosphoinositide cleavage by phospholipase C. Physiological effects in permeabilized platelets and Limulus photoreceptor cells. J Biol Chem. 1985 Nov 5;260(25):13496–13501. [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]