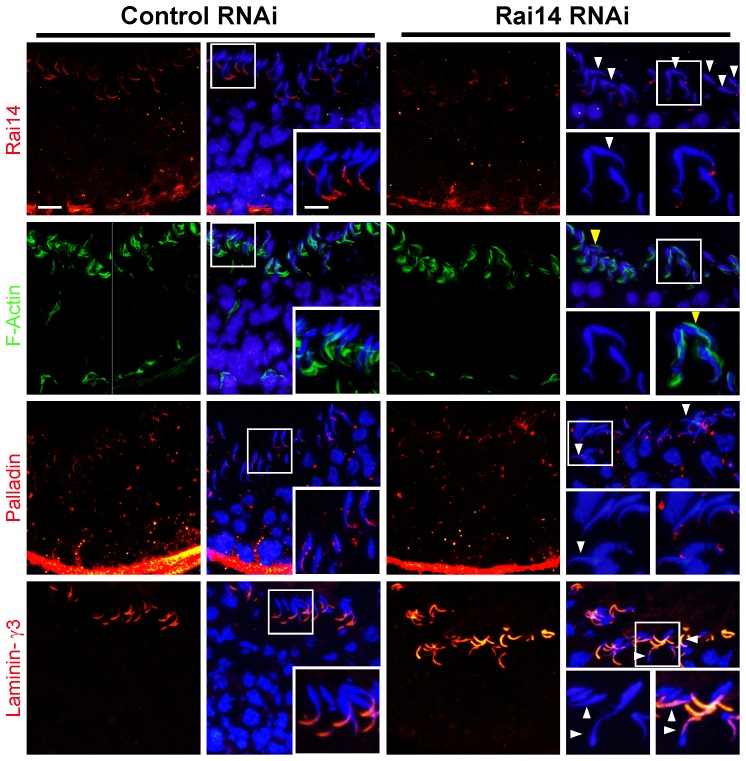
Figure 7. Effects of Rai14 knockdown in the testis on F-actin organization and protein distribution at the apical ES.
Following a knockdown of Rai14 in the testis, a considerable loss of Rai14 signals (red fluorescence) were detected in the seminiferous epithelium of stage VII tubules as illustrated in this tubule here, in particular at the apical ES site. As shown in the Rai14 RNAi stage VII tubule, Rai14 was virtually not detected at the apical ES surrounding the heads of these step 19 spermatids, and polarity was disrupted in many of these spermatids lacking Rai14 since mis-oriented spermatids no longer pointing toward the basement membrane (see “white” arrowheads), perhaps due to the loss of actin filament bundles at the apical ES, which likely required Rai14 for their maintenance. This notion was strengthened when F-actin (green fluorescence) was visualized by FITC-conjugated phalloidin in Rai14 RNAi vs. control group. In control testes, at stage VII, F-actin was restricted to the tip of the spermatid head, mostly to the concave side of the head. After Rai14 knockdown, F-actin no longer restricted to the concave side of the spermatid head, instead considerable F-actin in many step 19 spermatids was found on the convex side of the spermatid head (see “yellow” arrowheads). This mis-localization of F-actin suggests that actin filament bundles in these spermatids were disrupted. Furthermore, while the signals of palladin (red fluorescence, an actin cross-linking and bundling protein), in the seminiferous epithelium in Rai14 knockdown group were not diminished vs. the control group, palladin was also found to become mis-localized, no longer found at the tips of the spermatid heads, but mis-localized and diffused away from spermatid head. Interestingly, the localization of laminin-γ3 chain (red fluorescence), an apical ES component of the integrin-laminin adhesion complex at the apical ES, was found not to be disrupted including mis-oriented spermatids with a loss of polarity. Scale bar = 20 µm, and scale bar in inset = 10 µm, which apply to other micrographs in the corresponding panel.