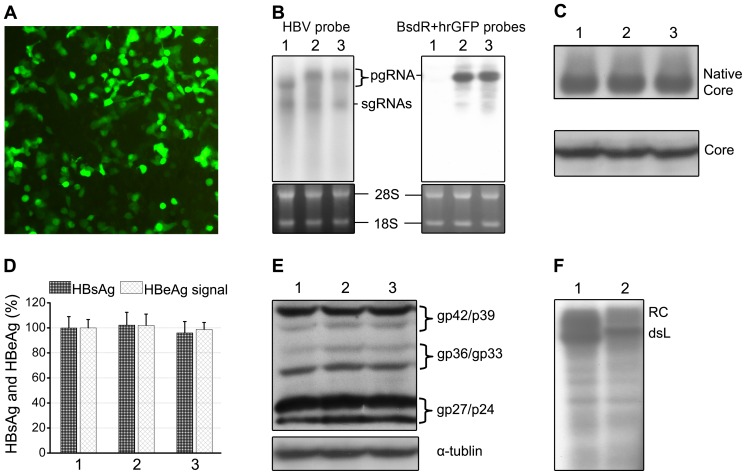
Figure 3. Evaluation of gene expression from the recombinant HBV vectors.
HepG2 cells were transfected with the wild-type HBV plasmid pCH-9/3093 (1), or the recombinant vector plasmids pCH-BsdR (2) or pCH-hrGFP (3). (A) Expression of the hrGFP transgene detected by immuno fluorescence. (B) HBV related transcripts. Total cytoplasmic RNA from the transfected cells was analyzed by Northern blotting using α 32P-labeled HBV probe (left panel) or a 1∶1 mixture of BsdR- and hrGFP-specific probes (right panel). The positions of the pgRNA and subgenomic (sg) RNAs are indicated. Ethidium bromide staining of 28 S and 18 S rRNA confirmed equal loading and intactness of the RNAs. No products attributable to major aberrantly spliced RNAs were detected by either probe. (C) Wild-type HBV-like production of core protein and capsids. Equal aliquots from cytoplasmic lysates were analyzed by immunoblotting with core-specific antibodies after NAGE (top; intact capsids) or after SDS-PAGE (bottom; core protein) and chemiluminescent detection. (D) HBsAg and HBeAg ELISA. Levels of secreted HBsAg and of HBeAg as surrogate marker for core protein were determined by ELISA and normalized those from the wild-type HBV plasmid pCH-9/3093 which were set as 100%. Values are the mean of three independent transfections; error bars indicate SD. Differences between groups were not significant (P>0.05). (E) Wild-type HBV-like production of L, M and S proteins. Immunoblotting after SDS-PAGE was performed using antibody 4/7B which recognizes a linear epitope in the S protein which thus is also contained in M and L proteins [71]. The positions of glycosylated and non-glycosylated L (gp42/p39) and S protein (gp27/p24), and of di-glycosylated and mono-glycosylated M protein (gp36/gp33) are indicated. Immunodetection of tubulin served as a loading control. (F) pCH-BsdR generates functional Pol. Cytoplasmic core particles from cells transfected with pCH-9/3093 and pCH-BsdR were subjected to endogenous polymerase reaction conditions including [α-32P]dCTP. Purified DNAs were separated by agarose gel electrophoresis, and radiolabeled products were detected by autoradiography. The positions of RC- and dsL DNA are indicated.