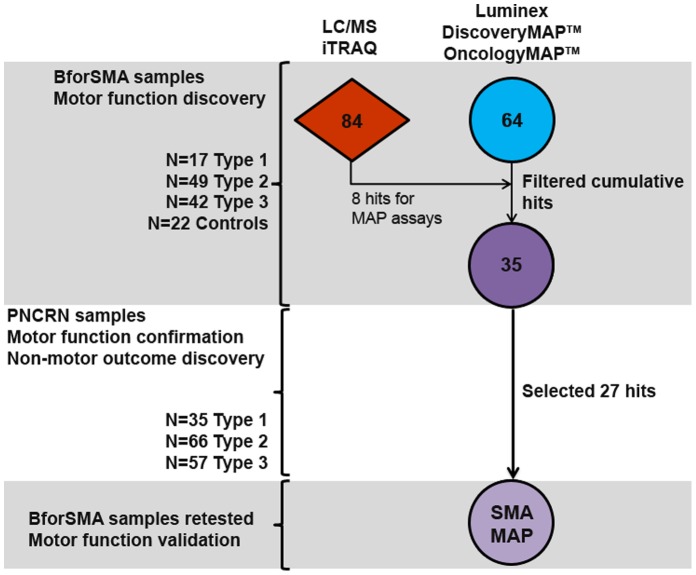
Figure 1. SMA plasma biomarker discovery campaign and confirmation schematic.
Analyte markers were identified in different discovery campaigns in two platforms. BforSMA samples were screened in LC/MS using iTRAQ technology, generating 84 markers that regressed with SMA motor function (MHFMS). Samples from the same study were screened in commercially available Luminex panels, yielding an additional 64 markers that regressed to motor function. There were 14 markers in the MAP panels that were hits in the LC/MS campaign, and 11 of these were repeat hits. New Luminex assays were created to represent the top 8 analytes from the LC/MS analysis. Filtering was performed by evaluation of statistical strength and assay performance, and 35 top analytes were selected for further MAP testing in a new sample set from the PNCRN natural history study. An additional 91 analytes were present in the panels for testing, allowing discovery based on non-motor outcome data that was collected in the PNCRN study. 13 analytes were repeat motor regressors, while 15 were new non-motor analytes. A total of 27 analytes were selected for inclusion to the final SMA-MAP panel, which was validated for reproducibility using unthawed samples from BforSMA.