Abstract
This study investigated the effects of imposed optical defocus on the expression patterns of bone morphogenetic protein 4 and 7 (BMP4, BMP7) in chick retinal pigment epithelium (RPE), as indicators of roles in postnatal eye growth regulation. BMP4 and BMP7 gene and protein expression patterns were characterized for retina, RPE and choroid tissues of young normal White-Leghorn chickens. The effects of short-term (2 and 48 h) exposure to monocular +10 and −10 diopter (D) lenses on RPE gene expression of BMP4 and BMP7 were also examined. Tissues from both treated and fellow eyes as well as from eyes of age-matched untreated birds were included in the latter experiment. Of ocular tissues comprising the posterior wall of the chick eye, RPE showed the highest expression of BMP4 and BMP7 mRNA, compared to retina and choroid. Western blots and immunohistochemisty confirmed the expression of BMP4 and BMP7 protein in all layers - retina, RPE, choroid and sclera. With imposed defocus, both BMP4 and BMP7 showed bidirectional changes in expression in RPE, however, with different temporal patterns. With +10 D lenses, BMP4 gene expression was up-regulated after both 2 and 48 h of treatment, while BMP7 expression was up-regulated only after 48 h of lens wear. With −10 D lenses, both BMP4 and BMP7 showed down-regulation of gene expression for both 2 and 48 h treatment durations. With the −10 D lens treatment applied for 48 h, gene expression for both BMP4 and BMP7 was also down-regulated in contralateral fellows of treated eyes compared to eyes of untreated chicks. The rapid changes in gene expression in chick RPE observed for both BMP4 and BMP7, up or down according to the sign of imposed optical defocus, resemble similar trends reported for BMP2. Further studies are needed to confirm the roles of BMPs as ocular growth modulators, as suggested by these data. The data also suggest a role for the RPE as a conduit for relaying growth modulatory retinal signals.
Keywords: myopia, retinal pigment epithelium (RPE), Bone Morphogenetic protein4 (BMP4), BMP7
INTRODUCTION
Myopia, the most common type of refractive error, has shown a rapid rise in prevalence worldwide in the past few decades, reaching epidemic levels in a number of Asian countries (Pan et al. 2012; Vitale et al. 2009).Most myopia is axial rather than refractive in nature, the product of excessive elongation of the vitreous chamber (McBrien and Millodot 1987; Whitmore 1992). Such changes are not without consequence - pathological complications such as retinal detachment, chorioretinal atrophy and neovascularization are associated with high myopia, classically defined as spherical equivalent refractive errors equal to or greater than − 6.0 D (Jones and Luensmann 2012; Saw et al. 2005). That the prevalence of high myopia is increasing along with the increase in overall myopia prevalence is thus a cause for great concern (Flitcroft 2012).
Both genetic and environmental factors are thought to play roles in the development of myopia (Flitcroft 2012); Wallman and Winawer 2004; Wojciechowski 2011). Studies with experimental animal models have provided convincing evidence for the role of environmental factors in the development of myopia and specifically, for the role of vision in early eye growth regulation (Wallman and Winawer 2004). In the presence of optical defocus, young eyes adjust their eye length in compensation, accelerating their growth in response to imposed hyperopic defocus and slowing it in response to myopic defocus. The net effects of these altered ocular growth patterns on the refractions of these eyes are induced myopia and hyperopia respectively. Data from neural lesioning studies involving optical defocus and others involving regionally localized imposed defocus suggest eye growth regulation to be largely localized to the eye itself (Choh et al. 2006; Diether and Schaeffel 1997; Smith et al. 2012; Wildsoet 2003). The study reported here addressed the hypothesis that the retinal pigment epithelium (RPE) is a critical component of the presumed retino-scleral signaling pathway mediating these eye growth changes (Rymer and Wildsoet 2005; Wallman and Winawer 2004). The RPE possesses a rich array of receptors, including ones for neurotransmitters already implicated in eye growth regulation (Buck et al. 2004; Fischer et al. 1998; Friedman et al. 1988; Rymer and Wildsoet 2005). Differential gene expression as well as morphological changes in RPE also have been documented during the development of myopia and hyperopia (Lin et al. 1993; Zhang Y et al. 2012; Zhang et al. 2012; Zhang et al. 2010).
Bone morphogenetic proteins (BMPs) are a large family of multifunctional growth factors that belong to the transforming growth factor-β superfamily, with important roles in osteogenesis and embryogenesis (Wagner et al. 2010). Based on amino acid sequence homology, BMP2 and BMP4 belong to one subgroup, being very similar to each other, while BMP7 belongs to another (Derynck and Miyazono 2008). Our recent finding of bidirectional regulation of BMP2 in response to optical defocus of opposite signs (Zhang et al. 2012), combined with the structural similarity between BMP2, BMP4, and BMP7 provided the principal motivation for the current study. Previous studies have also linked both BMP4 and BMP7 to embryonic eye morphogenesis as well as diseases of the adult retina (Fischer et al. 2004; Lyons et al. 1995; Mathura et al. 2000; Muller et al. 2007; Xu et al. 2012). For example, a genetics study using a loss-of function mutation of BMP7 in mouse reported severe defects in the developing eye (Dudley et al. 1995). Another human genetics study proposing BMP4 as a possible candidate gene for myopia provided additional motivation for the current study (Bakrania et al. 2008).
In the study reported here, we found BMP4 and BMP7 to be expressed at the gene and protein levels in retina, RPE and choroid of young chicks. We also report that like BMP2, BMP4 and BMP7 show similar optical defocus sign-dependent, bidirectional regulation in chick RPE although differences in temporal patterns of expression were also observed between BMP4 and BMP7.
MATERIALS and METHODS
Animals & Lens Treatments
White-Leghorn chickens were obtained from a commercial hatchery (Privett, Portales, NM), and raised under a 12-h light/12-h dark cycle. Experiments were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and approved by the Animal Care and Use Committee (ACUC) at University of California, Berkeley, CA. For the RPE real-time PCR study described below, chicks wore monocular +10 or −10 D lenses from 19 days of age for 2 or 48 h. Data for the four treatment groups represent the compilation of four independent repetitions of this experiment (4 ~ 6 chicks in each experiment). Age-matched untreated chicks also were included as additional controls in this experiment. The effects of these lens treatments on ocular dimensions have been reported previously (Zhang et al. 2012); in brief, the positive lens induces choroidal thickening and the negative lens, choroidal thinning, the changes being larger with the longer treatment period and in each case contributing to changes in vitreous chamber lengths (shortening and lengthening respectively).
Tissue Sample Collection for RNA and Protein Studies
Retina, RPE, and choroid samples collected from 19-day and 21-day old untreated chicks were used to study normal BMP4 and BMP7 gene and protein expression. RPE samples also were collected from age-matched lens-treated as well as untreated chicks for a real-time PCR study. The method of sample collection was as described previously (Zhang et al. 2012). Briefly, chicks were sacrificed, eyes enucleated and then retina, RPE, and choroid isolated and collected. For RNA samples, all three ocular tissues were lysed with RLT buffer from RNeasy Mini kits (Qiagen, Valencia, CA). Ocular protein samples were lysed with RIPA buffer (Sigma-Aldrich, St. Louis, MO) containing a protease inhibitor cocktail (Sigma-Aldrich). All samples were stored at −80 °C immediately for later use.
RNA Purification and Real-Time PCR
For retina and RPE samples, total RNA was purified using RNeasy Mini kits, while RNA from choroid samples was purified using an RNeasy Fibrous Tissue Mini Kit (Qiagen). On-column DNase digestion was performed for RNA samples from all three tissues. RNA concentration and optical density ratio of A260/A280 were measured on a spectrophotometer (NanoDrop 2000), NanoDrop Technologies, Inc., Wilmington, DE).
Total RNA (0.4 µg) was reverse transcribed to cDNA (SuperScript III First-Strand Synthesis System for RT-PCR, Invitrogen, Carlsbad, CA). The amount cDNA used in each PCR reaction varied across tissues and between genes, according to expression levels. Primers for BMP4 and BMP7 were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA. Table 1). Chick glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene (Zhang et al. 2012). The efficiency (E) of primers was calculated using equation (1), with the slope of the standard curves generated using ten-fold serial dilutions of cDNA. Mean normalized expression (MNE) was calculated for target genes using equation (2) (Simon 2003), and mean mRNA expression levels calculated using equations (3). Lens treatment-induced changes were expressed as Fold-changes, calculated using equation (4). QuantiTect SYBR Green PCR Kits (Qiagen) were used for mRNA amplification with a StepOnePlus Real-Time PCR System (Applied Biosystems). Melt curves were examined to verify the yield of single peak products. All real-time PCR reactions were performed in triplicate.
| (1) |
| (2) |
| (3) |
| (4) |
Table 1.
Primer information for chick BMP4 and BMP7
| Gene | NCBI Access Number | Sequences (5’-3’) | Efficiency | Amplicon |
|---|---|---|---|---|
| BMP4 | NM_205237.2 | Forward: 5’-CCAGCAAATCAGCCGTCAT-3’ | 97.5 % | 57 bp |
| Reverse:5’-CGGACTGGAGCCGGTAGA-3’ | ||||
| BMP7 | XM_417496.3 | Forward:5’-GGTGGCAGGACTGGATCATC-3’ | 100 % | 64 bp |
| Reverse:5’-GCGCATTCTCCTTCACAGTAATAC-3’ |
Western Blots
Protein expression profiling was limited to retina, RPE, and choroid samples from untreated chicks. A BCA assay (Pierce Biotechnology, Rockford, IL) was used to measure total protein concentration in all samples. Samples (40 µg) were electrophoresed on 4–12% gradient gels (NuPAGE 4–12% Bis-Tris Gel, Invitrogen) under reducing conditions, before being transferred to nitrocellulose membranes (iBlot Gel Transfer Stacks, Invitrogen). The reducing conditions involved processing with LDS sample buffer (Invitrogen) and dithiothreitol (DTT), and heating at 95 °C for 5 minutes. Membranes were blocked (StartingBlock T20 [TBS]; Pierce Biotechnology) and then incubated with either a mouse anti-human monoclonal antibody against BMP4 (# ab93939, Abcam, San Francisco, CA), or a rabbit anti-human polyclonal antibody against BMP7 (# ab93636, Abcam). Blots were subsequently labeled with HRP-conjugated goat anti-mouse IgG (# 31430, Pierce Biotechnology) or goat anti-rabbit IgG (# AP132P, Millipore). Immunoreactive bands were detected with chemiluminescence (Supersignal Pico ECL; Pierce Biotechnology) and imaged with a bioimaging system (FluorChem Q, Alpha Innotech; San Leandro, CA). Commercial mature BMP4 protein (# ab87063, Abcam) and mouse brain lysates were used as positive controls for testing the BMP4 antibody, while human kidney lysates (# ab30203, Abcam) and mouse brain lysates were used as positive controls for BMP7 antibody. Assays involved three separate biological samples and triplicate repeats. For BMP4, there is 95.7% similarity between chick and mouse, and chick and human sequences. For BMP7, the equivalent figures are 99.3% and 98.6% respectively.
Immunohistochemistry
Posterior eyecups were prepared from freshly enucleated eyes by removing the anterior segments (cornea and lens); the eyecups were then immersed in optimal cutting temperature (OCT) compound (Ted Pella Inc., Redding, CA) and stored at −80 °C immediately for later use. Seven µm cryostat sections were prepared and dried at room temperature. Sections were then fixed with acetone, washed with PBS, and blocked with 10% normal goat serum. A mouse anti-human monoclonal antibody against BMP4 (# ab93939, Abcam), rabbit anti-human polyclonal antibody against BMP7 (# ab93636, Abcam), or isotype control (Invitrogen) was used for immunostaining overnight at 4 °C. Sections were subsequently labeled with Alexa Fluor 546 goat anti-mouse or goat anti-rabbit IgG conjugated secondary antibody (Invitrogen), and counterstained with 4',6-diamidino-2-phenylindole (DAPI; Vector Labs, Burlingame, CA). Negative controls were included; sections were incubated in mouse and rabbit isotype IgG and then the secondary antibody. Sections were imaged with a Zeiss microscope (Zeiss Axioplan 2 Imaging, Carl Zeiss, Inc., Oberkochen, Germany). Staining was performed on three biological repeats.
Statistical Analysis
Paired Student’s t-tests were used to compare gene expression in the RPE from lens-treated eyes with their fellow eyes. To look for interocular yoking as previously observed under similar experimental conditions with BMP2 expression (Zhang et al. 2012), one-way ANOVAs with post-hoc testing (Fisher’s least significant difference, LSD) were used to compare gene expression data for eyes of untreated chicks, treated and contralateral fellow eyes of lens-treated chicks.
RESULTS
Expression of BMP4 and BMP7 in Normal Chick Ocular Tissues
mRNA Expression of BMP4 and BMP7
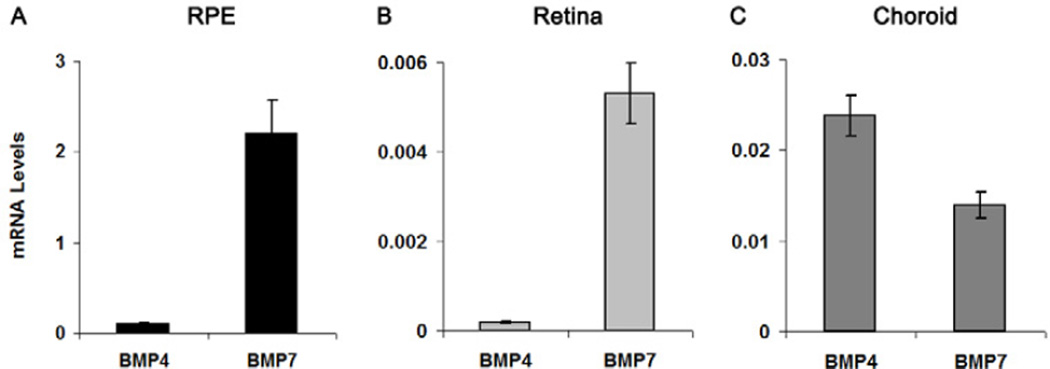
BMP4 and BMP7 mRNA was found to be expressed in chick retina, RPE, and choroid (Fig. 1). BMP4 and BMP7 were both highly expressed in RPE (BMP4, 0.10 ± 0.01, n = 38; BMP7, 2.20 ± 0.37, n = 32; Figure 1A), with BMP7 showing higher expression than BMP4. Expression levels of BMP4 and BMP7 in retina and choroid were lower, although the retinal data a similar bias towards higher expression of BMP7. In retina, BMP4 and BMP7 mRNA levels were 0.0002 ± 0.00002 (n = 20) and 0.005 ± 0.0007 (n = 12), respectively (Fig 1B). In choroid, BMP4 and BMP7 mRNA levels were 0.024 ± 0.002 (n = 16) and 0.014 ± 0.001 (n = 8), respectively (Fig. 1C). In an earlier study (Zhang et al. 2012), we also found differences in baseline expression levels of GAPDH in these tissues, the housekeeping gene used in these analysis (retina:RPE:choroid = 15:3:1), although the latter differences do not account for the differences in BMP4 and BMP7 expression between these tissues.
Figure 1.
mRNA levels of BMP4 and BMP7 in chick RPE (A), retina (B), and choroid (C). Data were expressed as mean ± SEM. GAPDH was housekeeping gene.
Protein Expression of BMP4 and BMP7
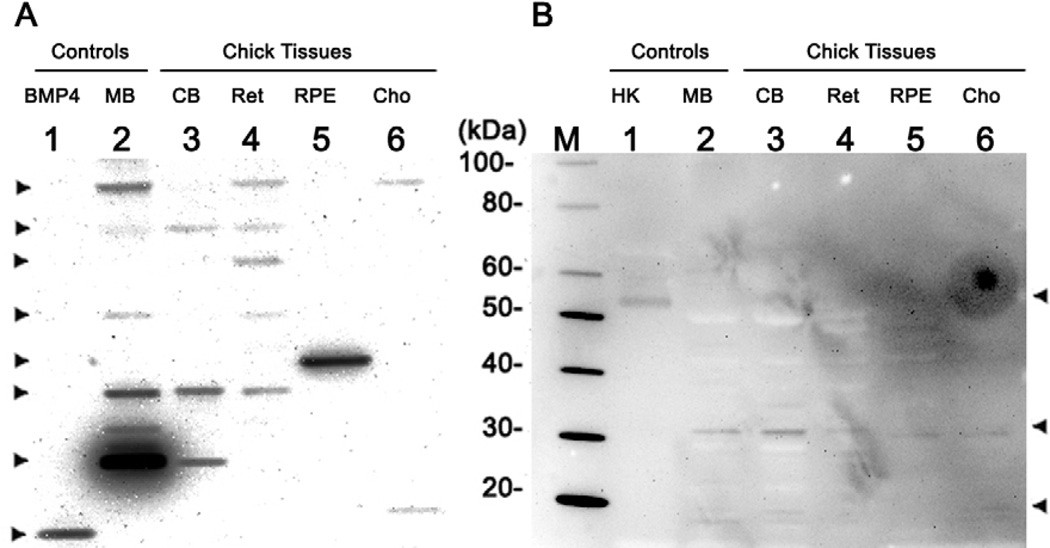
Both BMP4 and BMP7 proteins were detected in Western blots prepared under reducing conditions from chick retina, RPE and choroid samples (Fig. 2A). In both blots, lane 1 and 2 were loaded with positive controls, while lane 3 – 6 were loaded with chick tissue samples. To understand the complicated patterns of BMP4 and BMP7 protein expression, it is important to note that monomers and dimers of the BMP proproteins and mature proteins, as well as other forms have been reported in published literature, a nd databases (in the public domain at http://www.uniprot.org/uniprot/Q90752), (Cui et al. 2001; Felin et al. 2010; Klosch et al. 2005; Monroe et al. 2000; Nelsen and Christian 2009). Masses corresponding to the different forms of BMP4 and BMP7 are listed in Table 2. Results of a database search (http://www.uniprot.org) and sequence analysis for the mature human, mouse, and chick BMP4 and BMP7 protein are provided in Supplement 1.
Figure 2.
Western blots showing protein expression profiles for BMP4 (A) and BMP7 (B) in retina, RPE, and choroid from adolescent chicks, prepared under reducing conditions. Lane M was loaded with protein marker. In blot A, lane 1 was loaded with denatured, reduced BMP4 protein (0.02 µg), lanes 2 and 3 with mouse brain and chick brain respectively, and lanes 4 to 6 with chick retina, RPE, and choroid, respectively. In blot B, lane 1 was loaded with human kidney lysates, and lanes 2 and 3 were loaded with mouse and chick brain, respectively. Lanes 4 to 6 were loaded with chick retina, RPE, and choroid, respectively. MB, mouse brain; CB, chick brain; Ret, retina; Cho, choroid; HK, human kidney lysates.
Table 2.
Masses (kDa) corresponding to different forms of BMP4 and BMP7, for three different species (aa: amino acid).
| Human | Mouse | Chick | ||||
|---|---|---|---|---|---|---|
| Proprotein | Mature Protein | Proprotein | Mature Protein | Proprotein | Mature Protein | |
| BMP4 | 46.6 (408 aa) | 13.1 (116 aa) | 46.5 (408 aa) | 13.2 (116 aa) | 46.5 (405 aa) | 12.8 (114 aa) |
| BMP7 | 49.3 (431 aa) | 15.7 (139 aa) | 49.2 (430 aa) | 15.6 (139 aa) | 49.5 (435 aa) | 15.7 (139 aa) |
For BMP4, the retina (lane 4, Fig. 2A) produced the most complex banding pattern. Two bands correspond to the dimer and monomer of the proprotein (~ 90 and ~ 46 kDa, respectively), and another band at ~ 35 kDa represented the dimer of mature protein. There also were two additional bands at ~ 60 and ~ 70 kDa, possibly representing the glycosylated proprotein. The choroid (lane 6) yielded only 2 bands, corresponding to the dimer of the proprotein (~ 90 kDa) and the monomer of mature BMP4 (~ 13 kDa; lane 1, which was loaded with commercial BMP4 protein, also showed single band at ~ 13 kDa). The RPE (lane 5) yielded the simplest pattern, with only one band, at ~ 40 kDa, which may represent a nuclear variant of BMP4 or mature BMP4, based on its molecular weight (Bessa et al. 2009; Felin et al. 2010; Klosch et al. 2005). The pattern of BMP4 expression showed variability both between individual chicks and between the 3 ocular tissues analyzed, with one bird showing negligible choroidal expression of BMP4. Samples prepared from mouse and chick brain show similar, albeit complex patterns. No obvious bands were detected in the negative control (data not shown).
The BMP7 protein also was detected in chick retina, RPE and choroid under reducing conditions (Fig. 2B). The retina (lane 4), RPE (lane 5), and choroid (lane 6) all showed bands at ~ 30 kDa, corresponding to the dimer of the mature protein. The RPE (lane 5) yielded an additional weak band at ~ 49 kDa, which represents the proprotein. The choroid (lane 6) showed another weak band at 15 kDa, corresponding to the monomer of mature BMP7. Human kidney lysates (lane 1), which served as a positive control, yielded a single band at ~ 50 kDa. Mouse and chick brain samples showed similar patterns (lane 2 and 3); each had two visible bands at ~ 30 and 15 kDa, corresponding to the dimer and monomer of mature BMP7, respectively. No obvious bands were detected in the negative control (data not shown).
Localization of BMP4 and BMP7 Protein
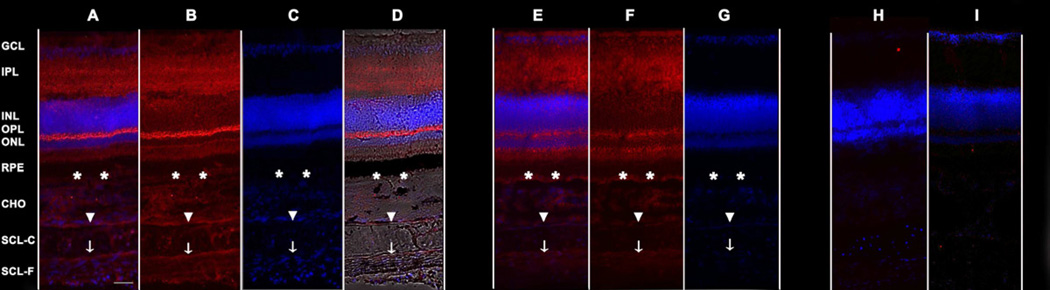
With immunohistochemistry labeling, expression patterns for BMP4 (Fig. 3A-D) and BMP7 (Fig. 3E-G) were found to be very similar; both were present in all layers of the posterior wall of the eye – retina, RPE, choroid, and sclera. In the retina, labeling for BMP4 and BMP7 was most intense and diffuse in the inner plexiform layer (IPL), outer plexiform layer (OPL), and outer segments of photoreceptors. Labeling was also observed in the ganglion cell layer (GCL), inner nuclear layer (INL), and outer nuclear layer (ONL), where both proteins appeared to be localized to the cytoplasm. In the RPE, BMP4 and BMP7 were evident in the basal side of cells, although the heavy pigmentation in the apical regions would tend to obscure labeling in this region. The choroid and sclera also showed labeling for both BMP4 and BMP7, diffuse in the case of the choroid while in the sclera, the fibrous layer exhibited more intense labeling than the cartilaginous layer in both cases. No obvious labeling was observed in negative controls (Fig. 3H, I).
Figure 3.
Sections from the posterior wall of the eyecup labeled for BMP4 and BMP7 (in red); nuclei were labeled with DAPI (in blue). BMP4 labeled sections (A-D), were either double-labeled for BMP4 and nuclei (A), labeled for BMP4 alone (B), or labeled for nuclei alone (C); double-labeling overlaid on unstained light microscopy image shown in D. BMP7 labeled sections (E-F) were either double-labeled for BMP7 and nuclei (E), labeled for BMP4 alone (F), or labeled for nuclei alone (G). Negative controls for BMP4 (H) and BMP7 (I) were imaged in blue and red channels. CHO, choroid; SCL-C, sclera cartilaginous layer; SCL-F, sclera fibrous layer. * Basal side of RPE, ▼ Inner boundary between choroid and sclera, ↓Border between cartilaginous and fibrous layers of sclera. Scale bar, 50 µM.
BMP4 and BMP7 Expression in RPE after Lens Treatments
Bidirectionally-regulated BMP4 and BMP7 mRNA Expression
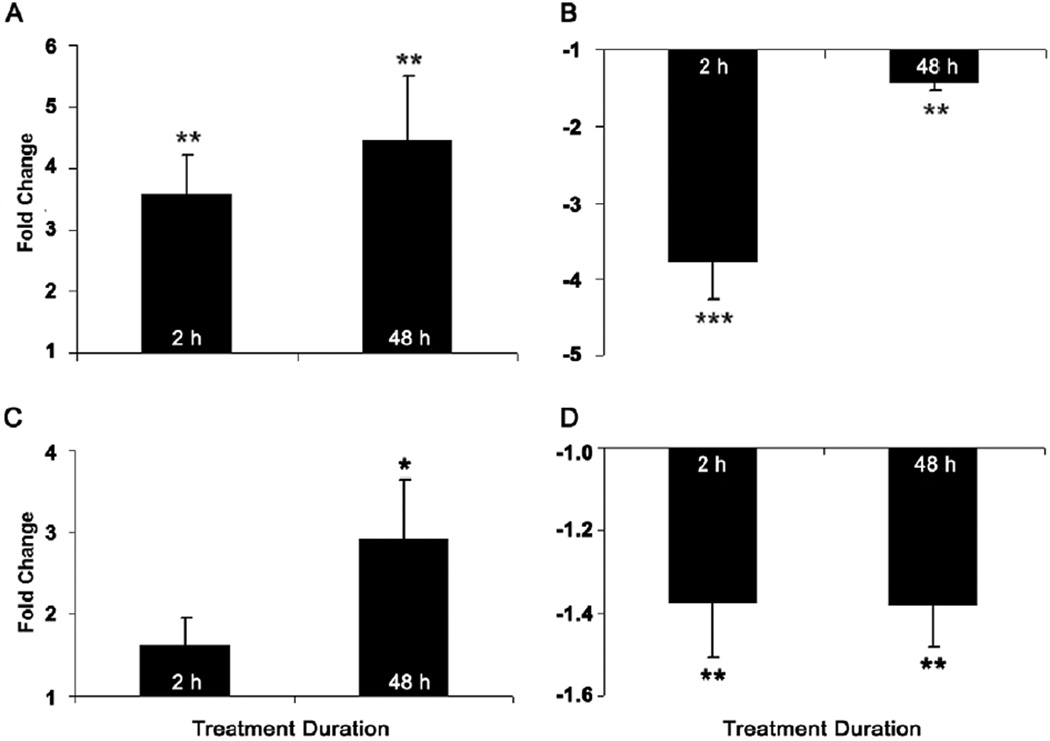
Both the +10 and −10 D lens treatments altered BMP4 gene expression, with the direction of change being defocus sign-dependent and consistent in direction for both treatment durations (Figs. 4A & B). Specifically, with the +10 D lenses, BMP4 gene expression was up-regulated by similar amounts after both 2 and 48 h of treatment, by 3.6- and 4.4-fold, respectively (p < 0.01, n = 18 in both cases; Fig. 4A). With the −10 D lenses, BMP4 gene expression was down-regulated, albeit slightly more with the shorter 2 h than the longer 48 h treatment period, by 3.8- and 1.4-fold, respectively (p < 0.001; p < 0.01, n = 19 for both cases; Fig. 4B). No significant interocular difference in BMP4 gene expression was observed in RPE from eyes of age-matched untreated birds (data not shown).
Figure 4.
Fold changes in mRNA levels of BMP4 (A, B) and BMP7 (C, D) in RPE after eyes exposed to defocus for 2 or 48 h via +10 D (A, C) and −10 D (B, D) lenses. Data were expressed as mean ± SEM. GAPDH was housekeeping gene. * p < 0.05, ** p < 0.01, *** p < 0.001.
While BMP7 also showed bidirectional changes in gene expression in response to optical defocus, the patterns differed in their temporal profiles from those observed with BMP4. Specifically, the +10 D lens induced 2.9-fold up-regulation of BMP7 only after 48 h of treatment (p < 0.05, n = 18. Fig. 4C); a shorter 2 h exposure to this lens did not affect gene expression (p > 0.05, n = 18). In contrast, gene expression of BMP7 was down-regulated with both 2 and 48 h of −10 D lens treatment, by 1.37- and 1.38-fold respectively (p < 0.01, n = 20; p < 0.01, n = 17; Fig. 4D). As expected, RPE from eyes of untreated age-matched birds showed no significant interocular differences in expression (right & left eyes compared; data not shown).
Yoking Effects of Lens Treatment on BMP4 and BMP7 Gene Expression
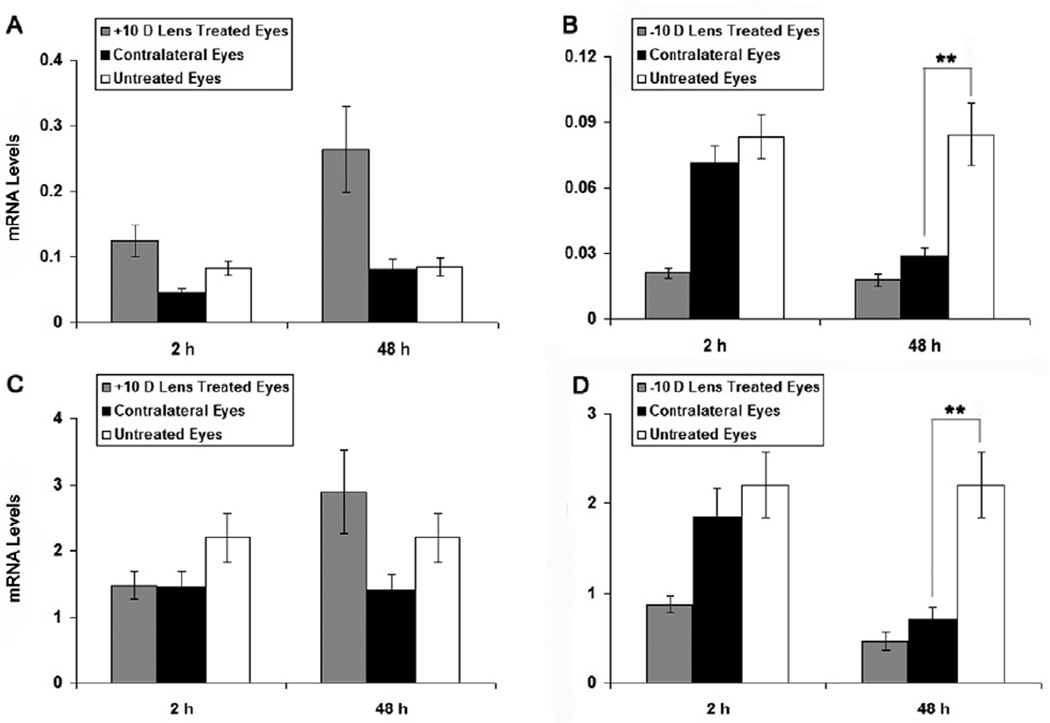
In our previous study of BMP2 gene expression in RPE, we observed significant changes in expression in the fellows to eyes wearing −10 D lenses and in the same direction as that observed in lens-wearing eyes, i.e. yoked expression (Zhang et al. 2012). Comparison of BMP4 and BMP7 expression in RPE from the fellows of the lens-treated eyes with values for RPE of eyes of untreated chicks in the current study revealed significant down-regulation of BMP4 and BMP7 expression in the fellows to eyes wearing −10 D lenses after 48 h of treatment (p < 0.01, Figs. 5B & D). No equivalent effect was observed with the +10 D lens treatments (Figs. 5A & C).
Figure 5.
BMP4 and BMP7 mRNA levels in RPE from eyes wearing +10 D (A, C) and - 10 D (B, D) lenses for 2 or 48 h, as well as from contralateral fellows to lens-treated eyes and eyes of untreated chicks. The fellows to eyes wearing −10 D lenses showed similar, albeit smaller down-regulation of BMP4 and BMP7 gene expression after 48 h of treatment. Data were expressed as mean ± SEM. GAPDH was housekeeping gene. ** p < 0.01.
DISCUSSION
The initial discovery that BMPs could induce ectopic bone and cartilage formation underlies the name of this family of growth factors (Urist 1965); Wozney et al. 1988). However, they are better described as multifunctional regulators, with influences on cell proliferation, differentiation, apoptosis and extracellular matrix accumulation, as well as on the development of many organs (Hogan 1996); Tominaga et al. 2011; Trousse et al. 2001). The current study investigated two BMPs, BMP4 and BMP7, which, at the protein level, were found to be present in all layers making up the back wall of the eye – retina, RPE, choroid and sclera, and at the gene level, were observed to be bidirectionally regulated in chick RPE in response to, and in accord with the sign of defocus. These results add to evidence from an earlier study of BMP2 from our laboratory, implicating the BMP family (TGF-β superfamily) in the defocus-driven modulation of eye growth (Zhang et al. 2012).
While BMP4 and BMP7 were observed to be both widely distributed across all of the layers enclosing the posterior vitreous chamber of the post-hatch chick eye, the RPE showed significantly higher gene expression than either retina or choroid. While these patterns suggest complex multifunctional ocular roles for these two growth factors, they also are consistent with the RPE playing a key regulatory role. Note that these expression patterns are also similar to those observed for BMP2 in our earlier study (Zhang et al. 2012). In functional terms, previous studies have suggested roles for BMPs in retinal patterning and differentiation (Behesti et al. 2006; Sakuta et al. 2006). In embryonic chick eyes, BMP4 mRNA expression was found to be initially restricted to the dorsal retina, with the expression pattern becoming less regionally localized and also weaker in later embryonic development; the distribution of BMP7 was more confined (Wordinger and Clark 2007). More mature retinas also express BMP4 and −7 although dorso-ventral asymmetries in expression are no longer apparent (Belecky-Adams and Adler, 2001). BMP7 has also been reported to enhance chick photoreceptor outer segment development in vitro (Sehgal et al. 2006).
Apart from studies in chicks, ocular studies of BMP4 and BMP7 expression are very limited in number. In the human embryonic eye, BMP4 expression has been observed in the optic vesicle, developing optic cup and developing lens (Bakrania et al. 2008; Wordinger and Clark 2007). Scleral expression of BMP4 has also been described for both guinea pig and adult human eyes (Cui et al. 2004; Wang et al. 2011). In the retina of the adult mouse, all cells were shown by in situ hybridization to express BMP4 and the RPE, to highly expressed it (Mathura et al. 2000). Similarly, BMP7 protein expression has been observed in all retinal layers in human, and the same study also identified similar BMP7 expression pattern in rat retina (Shen et al. 2004). These results are similar as results reported here for the chick retina.
In the context of ocular growth regulation and myopia, the most significant finding from our study is the apparent optical defocus, sign-dependent bidirectional regulation of BMP4 and BMP7 gene expression in chick RPE. Specifically, BMP4 and BMP7 gene expression showed significant up-regulation in response to imposed myopic defocus (+10 D lens treatment), while expression of both genes was down-regulated with imposed hyperopic defocus (−10 D lens treatment). These patterns of up- and down-regulation of BMP4 and BMP7 expression correspond to slowed and accelerated ocular elongation, respectively. In the case of BMP4, changes in gene expression were seen soon after treatments were initiated (i.e., at 2 h), ruling out the possibility that the changes were a byproduct of altered ocular growth, which would have been minimal at this time (Zhang et al. 2012). With both lens treatments and both genes, the early changes in expression persisted out to 48 h of treatment, the apparent decline in the response to the −10 D lenses after 48 h reflecting the similar, albeit smaller change in fellow eyes, rather than a real decline in expression. These more prolonged changes in gene expression tend to argue against them being simply responses to the altered visual (retinal defocus) conditions. Instead the results raise the possibility that BMP4 and BMP7 are themselves, negative growth modulators, acting on the choroid and/or sclera. In line with this proposal, the addition of exogenous BMP4 is reported to alter eye shape and reduce eye size in the mouse embryo (Behesti et al. 2006). There has been no equivalent study in chicks although intraocular injection of BMP4, given before treatment with a neuron toxin, is reported to suppress the proliferation of Müller glia and thus reduce retina damage in young chicks (Fischer et al. 2004). In two separate studies involving cultured human scleral fibroblasts, BMP2 was shown to promote cell proliferation and differentiation and also alter the expression of key extracellular matrix (ECM) genes and proteins (Hu et al. 2008; Wang et al. 2011). Assuming that differentially expressed BMP genes in RPE are translated into protein, and subsequently secreted into the choroid, these BMPs may affect changes in ocular dimensions through changes in ECM remodeling in either or both the choroid and sclera (Derynck and Miyazono 2008; Reddi 2000). These possibilities will be explored in future studies.
The current findings of bidirectionally regulated gene expression by optical defocus, of BMP4 and BMP7 in RPE closely resemble our finding for BMP2 in an earlier, recently reported study (Zhang et al. 2012). While BMP2, BMP4 and BMP7 are closely related, structurally (Derynck and Miyazono 2008; Goldman et al. 2009; Kawabata et al. 1998), and all three BMPs activate the same subtypes of BMP receptors (Wagner et al. 2010), studies of embryonic eye development in the chick describe spatially and temporally distinct expression patterns for these BMPs (Belecky-Adams and Adler 2001; Francis et al. 1994). Nonetheless, the latter observations are consistent with general behavior of these growth factors; their effects on developmental regulation, cell proliferation and differentiation can be similar or different, depending on the developmental stage and cell type (Chalazonitis et al. 2004; Mathura et al. 2000; Wang et al. 2011). The close similarity of the RPE gene expression profiles of BMP2, BMP4 and BMP7 for the optical defocus conditions imposed in our studies suggests that these three BMPs are co-regulated and/or may play similar roles in refractive error and eye growth regulation.
The experimental myopia literature contains many examples of interocular yoking involving gene expression, as well as refractive error and ocular growth rate changes in response to lenses and drug treatments (Buck et al. 2004; Fischer et al. 1999; Ohngemach et al. 2004; Schippert et al. 2006; Siegwart and Norton 2002; Stone et al. 2003; Wildsoet and Wallman 1995). Further examples are provided by our findings for both BMP4 and BMP7, that gene expression was significantly down-regulated in both −10 D lens-treated eyes and their fellows after 48 h of lens wear. This interocular yoking phenomenon was not evident with either the shorter 2 h exposure for the −10 D lens treatment or with either the 2 or 48 h + 10 D lens treatments. In our earlier study, we observed significant interocular yoking of gene expression changes for BMPR2 and a hint of similar yoking for BMP2 with −10 D lenses, although the latter effect was not statistically significant (Zhang et al. 2012). While the mechanisms underlying such interocular yoking remain to be elucidated, there appear to be treatment- and gene-dependent differences that need to be accounted for. For example, for BMP2, BMP4 and BMP7, the yoking effects on RPE gene expression was limited to our negative lens treatment, while yoking of ZENK protein expression in retina has been reported with both positive and negative lens treatments, as well as with recovery from form deprivation myopia (Bitzer and Schaeffel 2002; Fischer et al. 1999). In a previous study, we found that sectioning of the optic nerve in chicks eliminated the inhibitory effect of monocular atropine on lens-induced myopia in fellow eyes (Xu et al. 2010). While neural feedback loops may also be involved in these yoked gene expression changes, a humoral mechanism is also plausible (Fischer et al. 1999).
Although it is now generally accepted that both genetic and environmental factors are likely to contribute to the development of human myopia, there appears little overlap in the genes implicated in human myopia and those linked to eye growth regulation in animal models of myopia (Morgan et al. 2012). Our findings implicating BMP4 in eye growth regulation in the chick represents a departure from this trend and a potentially important break-through, with BMP4 being recently put forward as a candidate gene for human myopia (Bakrania et al. 2008). The latter study reported a mutation in BMP4 in a range of subjects presenting with anophthalmia, microphthalmia, or myopia although a causal link is yet to be established. In terms of animal studies, also of potential relevance is the finding in zebrafish of severe myopia linked to a mutation of low density lipoprotein receptor-related protein 2 (lrp2), which serves as an endocytic receptor for BMP4, in addition to other bioactive molecules (Veth et al. 2011). These observations lend weight to our data suggesting that BMP4 plays an important role in eye growth regulation.
In summary, we found in young chicks BMP4 and BMP7 mRNAs and proteins to be expressed throughout the tissues making up the posterior ocular wall, implying that they serve important regulatory functions in these tissues. Key ocular growth modulatory roles for these growth factors and a key role of the RPE as a signal transducer or relay in the related retino-scleral cascade are suggested by the further observations of sign-dependent, optical defocus-induced changes in gene expression in the RPE for both BMPs. Further investigations of these growth factors as possible novel myopia control treatments are warranted.
Supplementary Material
Supplement 1. Amino acid sequence alignment for human, mouse, and chick BMP4 (A) and BMP7 (B), prepared using ClustalW2, with amino acid sequences obtained from database UniProt Knowledgebase (UniProtKB): human BMP4 (P12644), mouse BMP4 (P21275), chick BMP4 (Q90752), human BMP7 (P18075), mouse BMP7 (P23359), and chick BMP7 sequences (Monroe et al. 2000). For BMP4, there is 95.7% similarity between chick and mouse, and chick and human sequences. For BMP7, the equivalent figures are 99.3 and 98.6%.
Highlights.
BMP4 and -7 are widely expressed in the posterior ocular tissues of young chicks.
RPE shows high BMP4 and -7 gene expression compared to retina and choroid.
BMP4 and -7 gene expression in RPE is sensitive to the sign of optical defocus.
RPE acts as a conduit for retinal growth signals targeting the choroid and sclera.
BMP4 and BMP7 are plausible ocular growth modulators in the chick eye.
ACKNOWLEDGMENT
The authors thank Professor Lu Chen and Don Yuen for their technical advice with immunohistochemistry and Western blot imaging (Vision Science Program, School of Optometry, University of California, Berkeley, CA). This study is supported by National Eye Institute Grants R01 EY012392 (CFW), T32 EY007043 (YZ), and K12 EY17269 (YL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bakrania P, Efthymiou M, Klein JC, Salt A, Bunyan DJ, Wyatt A, Ponting CP, Martin A, Williams S, Lindley V, Gilmore J, Restori M, Robson AG, Neveu MM, Holder GE, Collin JR, Robinson DO, Farndon P, Johansen-Berg H, Gerrelli D, Ragge NK. Mutations in BMP4 cause eye, brain, and digit developmental anomalies: overlap between the BMP4 and hedgehog signaling pathways. Am J Hum Genet. 2008;82:304–319. doi: 10.1016/j.ajhg.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behesti H, Holt JK, Sowden JC. The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup. BMC Dev Biol. 2006;6:62. doi: 10.1186/1471-213X-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belecky-Adams T, Adler R. Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol. 2001;430:562–572. [PubMed] [Google Scholar]
- Bessa PC, Cerqueira MT, Rada T, Gomes ME, Neves NM, Nobre A, Reis RL, Casal M. Expression, purification and osteogenic bioactivity of recombinant human BMP-4, -9, -10, -11 and -14. Protein Expr Purif. 2009;63:89–94. doi: 10.1016/j.pep.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Bitzer M, Schaeffel F. Defocus-induced changes in ZENK expression in the chicken retina. Invest Ophthalmol Vis Sci. 2002;43:246–252. [PubMed] [Google Scholar]
- Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthalmol Vis Sci. 2004;45:402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, D'Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. doi: 10.1523/JNEUROSCI.3688-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choh V, Lew MY, Nadel MW, Wildsoet CF. Effects of interchanging hyperopic defocus and form deprivation stimuli in normal and optic nerve-sectioned chicks. Vision Res. 2006;46:1070–1079. doi: 10.1016/j.visres.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Cui W, Bryant MR, Sweet PM, McDonnell PJ. Changes in gene expression in response to mechanical strain in human scleral fibroblasts. Exp Eye Res. 2004;78:275–284. doi: 10.1016/j.exer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Cui Y, Hackenmiller R, Berg L, Jean F, Nakayama T, Thomas G, Christian JL. The activity and signaling range of mature BMP-4 is regulated by sequential cleavage at two sites within the prodomain of the precursor. Genes Dev. 2001;15:2797–2802. doi: 10.1101/gad.940001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Miyazono K. The TGF-β family. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2008. pp. 1–49. [Google Scholar]
- Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Felin JE, Mayo JL, Loos TJ, Jensen JD, Sperry DK, Gaufin SL, Meinhart CA, Moss JB, Bridgewater LC. Nuclear variants of bone morphogenetic proteins. BMC Cell Biol. 2010;11:20. doi: 10.1186/1471-2121-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999;2:706–712. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998;392:273–284. [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004;27:531–542. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622–660. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Francis PH, Richardson MK, Brickell PM, Tickle C. Bone morphogenetic proteins and a signalling pathway that controls patterning in the developing chick limb. Development. 1994;120:209–218. doi: 10.1242/dev.120.1.209. [DOI] [PubMed] [Google Scholar]
- Friedman Z, Hackett SF, Campochiaro PA. Human retinal pigment epithelial cells possess muscarinic receptors coupled to calcium mobilization. Brain Res. 1988;446:11–16. doi: 10.1016/0006-8993(88)91291-7. [DOI] [PubMed] [Google Scholar]
- Goldman DC, Donley N, Christian JL. Genetic interaction between Bmp2 and Bmp4 reveals shared functions during multiple aspects of mouse organogenesis. Mech Dev. 2009;126:117–127. doi: 10.1016/j.mod.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hu J, Cui D, Yang X, Wang S, Hu S, Li C, Zeng J. Bone morphogenetic protein-2: a potential regulator in scleral remodeling. Mol Vis. 2008;14:2373–2380. [PMC free article] [PubMed] [Google Scholar]
- Jones D, Luensmann D. The prevalence and impact of high myopia. Eye Contact Lens. 2012;38:188–196. doi: 10.1097/ICL.0b013e31824ccbc3. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Klosch B, Furst W, Kneidinger R, Schuller M, Rupp B, Banerjee A, Redl H. Expression and purification of biologically active rat bone morphogenetic protein-4 produced as inclusion bodies in recombinant Escherichia coli. Biotechnol Lett. 2005;27:1559–1564. doi: 10.1007/s10529-005-1794-x. [DOI] [PubMed] [Google Scholar]
- Lin T, Grimes PA, Stone RA. Expansion of the retinal pigment epithelium in experimental myopia. Vision Res. 1993;33:1881–1885. doi: 10.1016/0042-6989(93)90015-o. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Mathura JR, Jr, Jafari N, Chang JT, Hackett SF, Wahlin KJ, Della NG, Okamoto N, Zack DJ, Campochiaro PA. Bone morphogenetic proteins-2 and -4: negative growth regulators in adult retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2000;41:592–600. [PubMed] [Google Scholar]
- McBrien NA, Millodot M. A biometric investigation of late onset myopic eyes. Acta Ophthalmol (Copenh) 1987;65:461–468. doi: 10.1111/j.1755-3768.1987.tb07024.x. [DOI] [PubMed] [Google Scholar]
- Monroe DG, Jin DF, Sanders MM. Estrogen opposes the apoptotic effects of bone morphogenetic protein 7 on tissue remodeling. Mol Cell Biol. 2000;20:4626–4634. doi: 10.1128/mcb.20.13.4626-4634.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- Muller F, Rohrer H, Vogel-Hopker A. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development. 2007;134:3483–3493. doi: 10.1242/dev.02884. [DOI] [PubMed] [Google Scholar]
- Nelsen SM, Christian JL. Site-specific cleavage of BMP4 by furin, PC6, and PC7. J Biol Chem. 2009;284:27157–27166. doi: 10.1074/jbc.M109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohngemach S, Buck C, Simon P, Schaeffel F, Feldkaemper M. Temporal changes of novel transcripts in the chicken retina following imposed defocus. Mol Vis. 2004;10:1019–1027. [PubMed] [Google Scholar]
- Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Morphogenetic messages are in the extracellular matrix: biotechnology from bench to bedside. Biochem Soc Trans. 2000;28:345–349. [PubMed] [Google Scholar]
- Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci. 2005;22:251–261. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- Sakuta H, Takahashi H, Shintani T, Etani K, Aoshima A, Noda M. Role of bone morphogenic protein 2 in retinal patterning and retinotectal projection. J Neurosci. 2006;26:10868–10878. doi: 10.1523/JNEUROSCI.3027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFbeta-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82:710–719. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Sehgal R, Andres DJ, Adler R, Belecky-Adams TL. Bone morphogenetic protein 7 increases chick photoreceptor outer segment initiation. Invest Ophthalmol Vis Sci. 2006;47:3625–3634. doi: 10.1167/iovs.06-0079. [DOI] [PubMed] [Google Scholar]
- Shen W, Finnegan S, Lein P, Sullivan S, Slaughter M, Higgins D. Bone morphogenetic proteins regulate ionotropic glutamate receptors in human retina. Eur J Neurosci. 2004;20:2031–2037. doi: 10.1111/j.1460-9568.2004.03681.x. [DOI] [PubMed] [Google Scholar]
- Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–2075. [PMC free article] [PubMed] [Google Scholar]
- Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- Smith EL, 3rd, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2012;51:3864–3873. doi: 10.1167/iovs.09-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RA, Liu J, Sugimoto R, Capehart C, Zhu X, Pendrak K. GABA, experimental myopia, and ocular growth in chick. Invest Ophthalmol Vis Sci. 2003;44:3933–3946. doi: 10.1167/iovs.02-0774. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Abe H, Ueda O, Goto C, Nakahara K, Murakami T, Matsubara T, Mima A, Nagai K, Araoka T, Kishi S, Fukushima N, Jishage K, Doi T. Activation of bone morphogenetic protein 4 signaling leads to glomerulosclerosis that mimics diabetic nephropathy. J Biol Chem. 2011;286:20109–20116. doi: 10.1074/jbc.M110.179382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–1301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Veth KN, Willer JR, Collery RF, Gray MP, Willer GB, Wagner DS, Mullins MC, Udvadia AJ, Smith RS, John SW, Gregg RG, Link BA. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010;2:mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao G, Xing S, Zhang L, Yang X. Role of bone morphogenetic proteins in form-deprivation myopia sclera. Mol Vis. 2011;17:647–657. [PMC free article] [PubMed] [Google Scholar]
- Whitmore WG. Congenital and developmental myopia. Eye (Lond) 1992;6(Pt 4):361–365. doi: 10.1038/eye.1992.74. [DOI] [PubMed] [Google Scholar]
- Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks--insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003;27:371–385. doi: 10.1076/ceyr.27.6.371.18188. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Bone morphogenetic proteins and their receptors in the eye. Exp Biol Med (Maywood) 2007;232:979–992. doi: 10.3181/0510-MR-345. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu Y, Wildsoet CF. The effect of optic nerve section on the yoked inhibition by atropine of lens-induced myopia in chicks. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 3677. [Google Scholar]
- Xu J, Zhu D, Sonoda S, He S, Spee C, Ryan SJ, Hinton DR. Over-expression of BMP4 inhibits experimental choroidal neovascularization by modulating VEGF and MMP-9. Angiogenesis. 2012;15:213–227. doi: 10.1007/s10456-012-9254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Ho Carol, Hammond D, CF W. Differential expression of BMP7, TGF-β2, and noggin in chick RPE after imposed optical defocus. Invest Ophthalmol Vis Sci. 2012;53 E-Abstract 3458. [Google Scholar]
- Zhang Y, Liu Y, Wildsoet CF. Bidirectional, Optical Sign-Dependent Regulation of BMP2 Gene Expression in Chick Retinal Pigment Epithelium. Invest Ophthalmol Vis Sci. 2012;53:6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Xu J, Nimri N, Wildsoet CF. Microarray analysis of RPE gene expression in chicks during long-term imposed hyperopic defocus. Invest. Ophthalmol. Vis. Sci. 2010;51 E-Abstract 3680. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1. Amino acid sequence alignment for human, mouse, and chick BMP4 (A) and BMP7 (B), prepared using ClustalW2, with amino acid sequences obtained from database UniProt Knowledgebase (UniProtKB): human BMP4 (P12644), mouse BMP4 (P21275), chick BMP4 (Q90752), human BMP7 (P18075), mouse BMP7 (P23359), and chick BMP7 sequences (Monroe et al. 2000). For BMP4, there is 95.7% similarity between chick and mouse, and chick and human sequences. For BMP7, the equivalent figures are 99.3 and 98.6%.