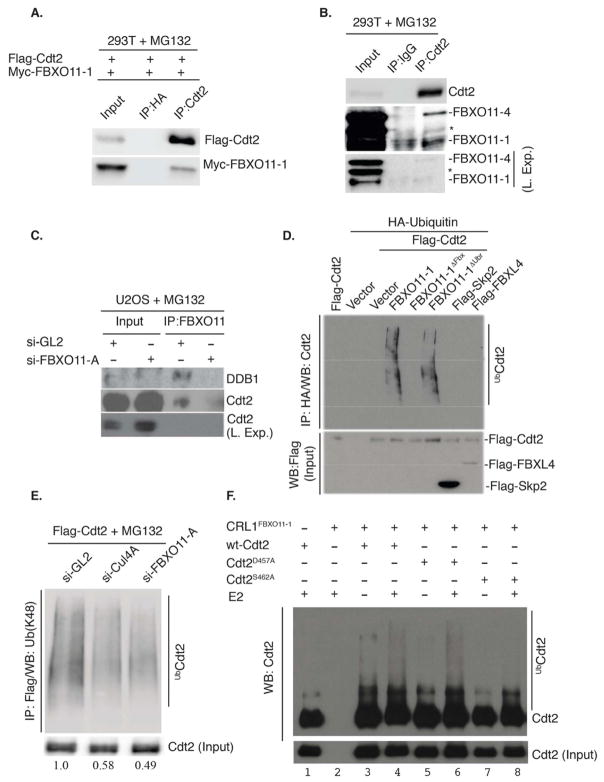
Figure 3. Cdt2 is a direct polyubiquitylation substrate of the CRL1FBXO11 E3 ligase.
(A) Immunoblot of Flag-FBXO11-1 co-immunoprecipitated with exogenous Cdt2 by anti-Cdt2 antibody from 293T cells transfected with plasmids expressing the indicated proteins. Cells were treated with MG132 for 3 hr prior to lysis.
(B) Similar to (A), except the interaction is observed with endogenous proteins. Both isoforms of FBXO11 (FBXO11-4 (upper) and FBXO11-1 (lower)) are detected in the anti-Cdt2 immunoprecipitates. Asterisk indicates a cross-reactive band in the anti-FBXO11 immunoblot. Two exposures are shown of the FBXO11 blot for clarity.
(C) Western blot showing that Cdt2 is detected in the anti-FBXO11 immunoprecipitates in control U2OS cells (si-GL2), but not in cells depleted of FBXO11 by si-FBXO11-A. DDB1, another component of the CRL4Cdt2 ubiquitin ligase, also co-immunoprecipitates with anti-FBXO11 in control cells, but not in cells depleted of FBXO11.
(D) FBXO11 promotes Cdt2 polyubiquitylation in vivo. Western blot analysis of polyubiquitylated Cdt2 (anti-Cdt2) in immunoprecipitated ubiquitylated proteins (anti-HA) from lysates of 293T cells transfected with the indicated F-box proteins, Flag-Cdt2 and HA-ubiquitin (see experimental procedure for details). Immunoblot of lysates with anti-Flag detects the expression of Flag-Cdt2, Flag-FBXL4 and Flag-Skp2 in the input.
(E) Depletion of 293T cells of FBXO11 or Cul4A decreases K-48-linked polyubiquitylation of Cdt2. 293T cells were transfected with flag-Cdt2 plasmid and subsequently transfected with the indicated si-RNA and treated with MG132 for 3 hr before lysis. Western blotting of anti-Flag-Cdt2 immunoprecipitates with anti-K48-linkage-specific-polyubiquitin antibody shows that both Cul4A and FBXO11 are required for maximal Cdt2 polyubiquitylation in vivo. Quantitation of polyubiquitylated Cdt2 relative to nonubiquitylated Cdt2 is shown below each lane.
(F) CRL1FBXO11 promotes the polyubiquitylation of Cdt2 in vitro. Immunoblot of Cdt2 immunoprecipitated from 293T cells transiently transfected with wt-Cdt2, Cdt2D457A or Cdt2S462A and incubated in an in vitro ubiquitylation reaction with separately immunopurified CRL1FBXO11-1 E3 ligase complex (prepared as described in Methods). Incubation was in the presence or absence of E2 ubiquitin-conjugating enzyme.