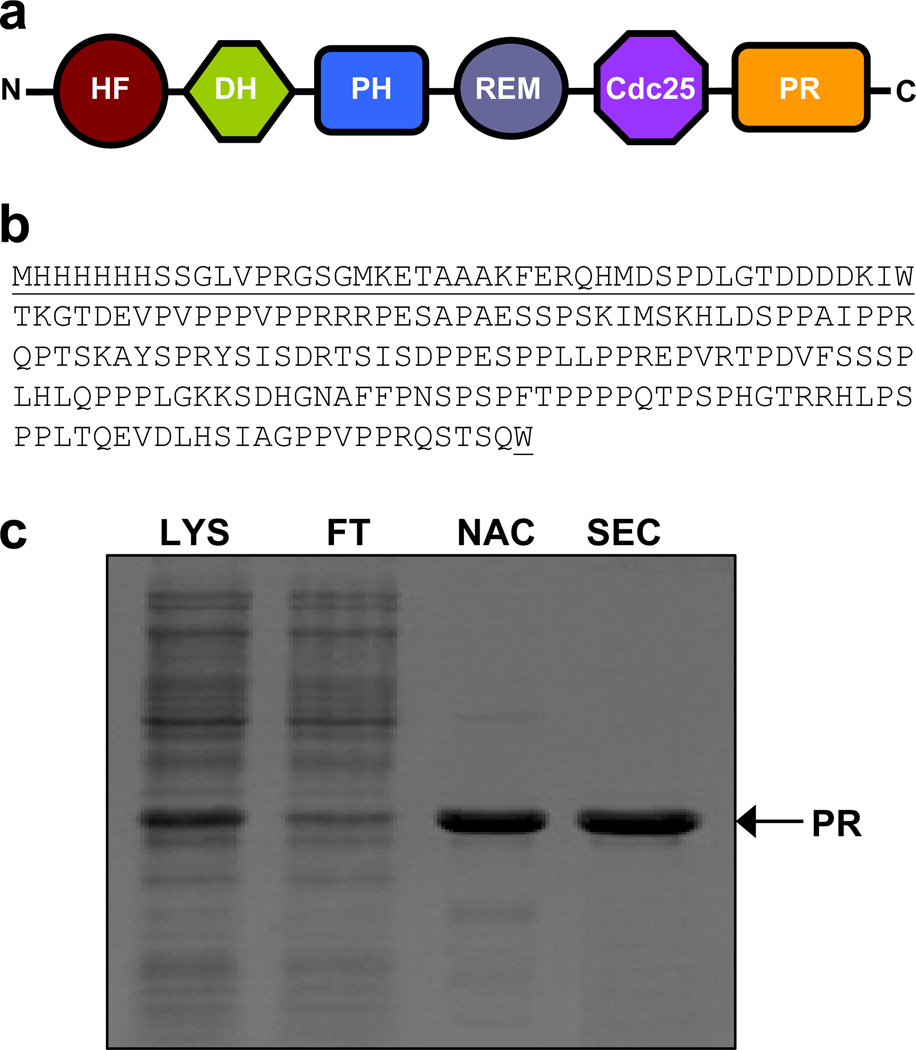
Figure 1.
Purification and characterization of the PR domain of Sos1. (a) Within Sos1 (residues 1–1333), the PR domain lies at the extreme C-terminal end. Other domains within Sos1 are HF (histone fold), DH (Dbl homology), PH (pleckstrin homology), REM (Ras exchange motif) and Cdc25 (cell division cycle 25). (b) Complete amino acid sequence of the recombinant PR domain (residues 1141–1300). The non-native amino acid residues introduced during cloning at both the N- and C-termini of the PR domain are underlined for clarity. (c) SDS-PAGE analysis of the PR domain. Briefly, total bacterial lysate (LYS) was loaded onto a Ni-NTA column, the flow-through (FT) was collected and after elution from the Ni-NTA affinity chromatography (NAC) column, the recombinant protein was further purified to apparent homogeneity by size-exclusion chromatography (SEC).