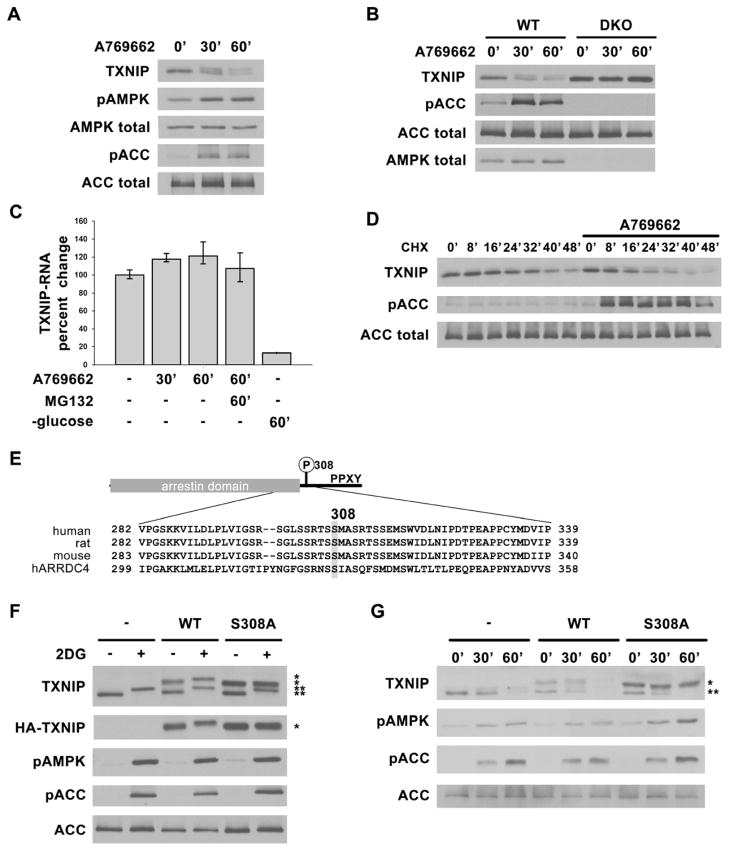
Fig. 2. AMPK phosphorylation of TXNIP on S308 accelerates its degradation.
Western blots showing decreasing TXNIP levels after AMPK activation in (A) primary rat hepatocytes and (B) AMPK WT and DKO MEFs treated with 1mM A769662 for 0′, 30′ and 60′. (C) qRT-PCR analysis of TXNIP mRNA from RNA isolated from HepG2 cells after various treatments indicating short-term activation of AMPK (e.g. using A769662) does not decrease TXNIP mRNA level while glucose starvation does as reported before. The values are the average of triplicates ±STDV. (D) HepG2 cells were pretreated with cycloheximide (CHX) for 20′ to stop protein synthesis, then stimulated with A769662 to activate AMPK. Lysates harvested at indicated time points show increased rate of TXNIP protein degradation with AMPK activation. (E) Domain structure of TXNIP and multiple sequence alignment of human, rat, mouse TXNIP and human ARRDC4 around Ser308. (F) HepG2 cells stably expressing vector control, HA-WT, or HA-S308A TXNIP treated with 25mM 2DG for 10′ show that S308A mutation abolishes the phosphorylation induced protein mobility upshift after AMPK activation. (G) HepG2 cells stably expressing vector control, HA-WT or HA-S308A TXNIP were treated with 1mM A769662 for 0′, 30′ and 60′. HA-S308A TXNIP protein is degraded at a slower rate than both HA-WT and endogenous TXNIP. *: HA-tagged protein, **: endogenous protein.