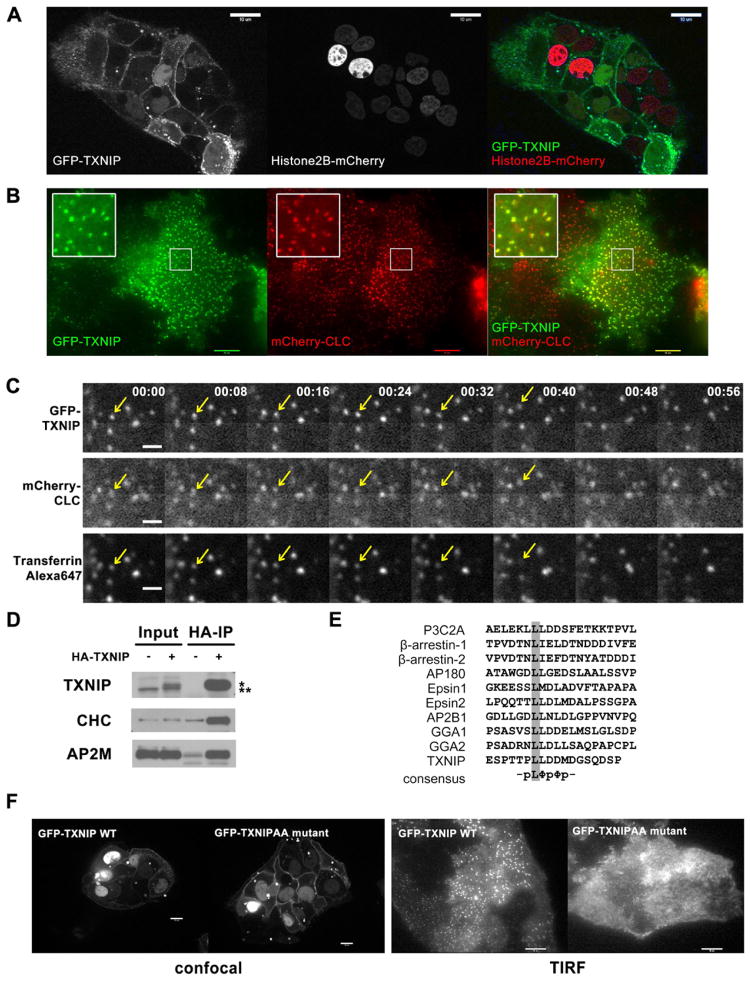
Fig. 3. Dual localization of TXNIP.
(A) Confocal live-cell images of HepG2 cells stably expressing GFP-TXNIP and Histone2B-mCherry, show plasma membrane localization of TXNIP in addition to its nuclear localization as reported before. (B) TIRF live-cell images of HepG2 cells stably expressing GFP-TXNIP and mCherry-clathrin light chain (CLC) show some TXNIP protein localization in clathrin-coated pits (CCP). (C) HepG2 cells stably expressing GFP-TXNIP and mCherry-CLC were labeled with Alexa647-transferrin at 4°C. After rinsing off excess transferrin, time-lapse images were taken at room temperature to capture endocytosis events using a confocal microscope. For every time point, there was a 2–3 seconds delay between each fluorophore: GFP was followed by mCherry and then Alexa647. The arrow points to an endocytosed CCP that contained both GFP-TXNIP and Alexa647-transferrin. This sequence is also shown in Video S3. The scale bar is 1 μM. (D) Western blot of HA IP of lysates from mouse liver expressing adenoviral HA-TXNIP probed with clathrin heavy chain (CHC) and adaptor AP2 μ subunit (AP2M) antibodies. *: HA-tagged protein, **: endogenous protein (E) Multiple sequence alignment of di-leucine motif in various clathrin-interacting proteins. (F) Confocal and TIRF live-cell images of HepG2 cells stably expressing GFP-WT or GFP-L351AL352A TXNIP show that the LL to AA mutation abolished TXNIP localization to the CCP, but not to the plasma membrane.