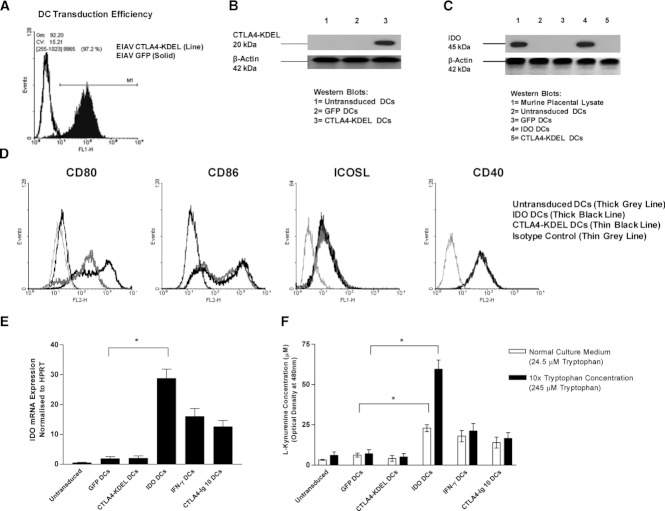
Figure 1.
DC transduction with EIAV-CTLA4-KDEL or EIAV-IDO. BM-derived BALB/c DCs were transduced with EIAV on day 6 of culture prior to LPS stimulation on day 8. (A) DC transduction efficiency was assessed by GFP expression using flow cytometry 72 h after transduction with EIAV-GFP or EIAV-CTLA4-KDEL (control). Expression of the (B) 20 kDa CTLA4-KDEL protein and (C) 45 kDa IDO protein in DC lysates was determined by western blotting 72 h after transduction with EIAV-CTLA-KDEL or EIAV-IDO, respectively, and compared with expression in EIAV-GFP-transduced or untransduced DCs and, in the case of IDO, murine placenta (positive control). Expression of the 42 kDa β-actin housekeeping protein was measured as a loading control. (D) Flow cytometry histograms show the surface expression of CD80, CD86, ICOSL, and CD40 on untransduced DCs, and DCs 72 h after transduction with EIAV-CTLA4-KDEL or EIAV-IDO (control). The results shown in (A–D) are representative of three independent experiments. (E–F) Untransduced, immature DCs were treated on day 7 with either IFN-γ or CTLA4-Ig. The DC culture media was supplemented with L-tryptophan on day 6 (final concentration, 245 μM), followed by LPS stimulation on day 8. (E) DCs were harvested on day 9 for quantitative PCR analysis to assess IDO mRNA expression. (F) IDO activity was assessed by a kynurenine assay using DC culture supernatants that were either supplemented with tryptophan or unsupplemented. Results are shown as the mean ± SD of triplicate wells and are representative of three independent experiments performed. * p < 0.05, two-tailed t-test.