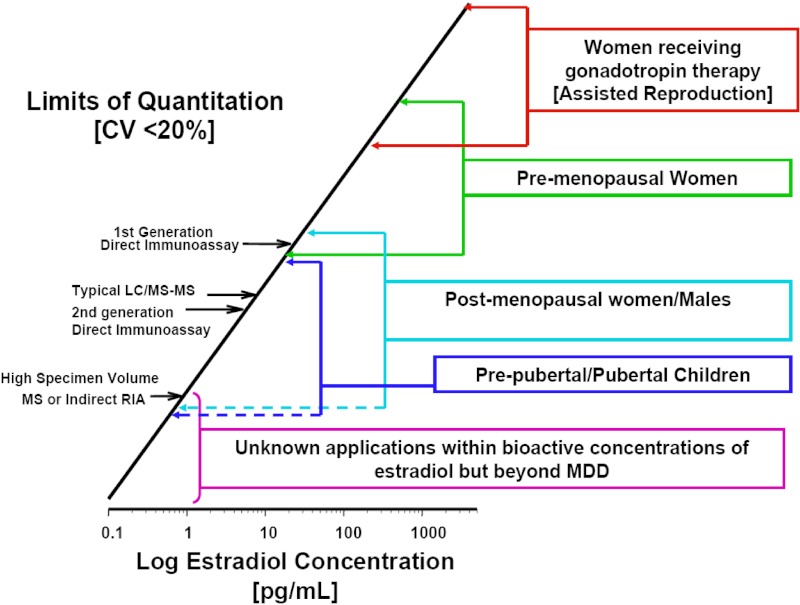
Figure 1.
Historical and current technical challenges to E2 assays with regard to analytical sensitivity. The primary methods used for clinical measurements are shown along the bar over a scale of E2 levels encompassing the situations in which measuring E2 in peripheral circulation is useful. (Detailed procedures for determining the limit of quantitation (LOQ) are available from the Clinical and Laboratory Standards Institute [CLSI EP17, http://www.abrf.org/index.cfm?method=list.getAttachment&disclaimerAck=1&msg=83913&att=688]). In this figure, a “rule-of-thumb” definition for the limit of quantitation (or minimal detectable dose [MDD]) is used; ie, the lowest concentration that can be measured with a coefficient of variation (CV) of 20% (19). It is useful to keep in mind that the 95% CI for a measure at this level is the mean ± 2 SD of repeated measurements. In other words, if the limit of quantitation is 80 pg/mL, values between 48 and 112 pg/mL cannot be distinguished with 95% confidence. The LOQ is typically dependent, among other things, on the operational condition of the instrument and the quality of reagents used; it therefore often differs in routine clinical laboratories when compared to the conditions under which the assay is first characterized and evaluated.