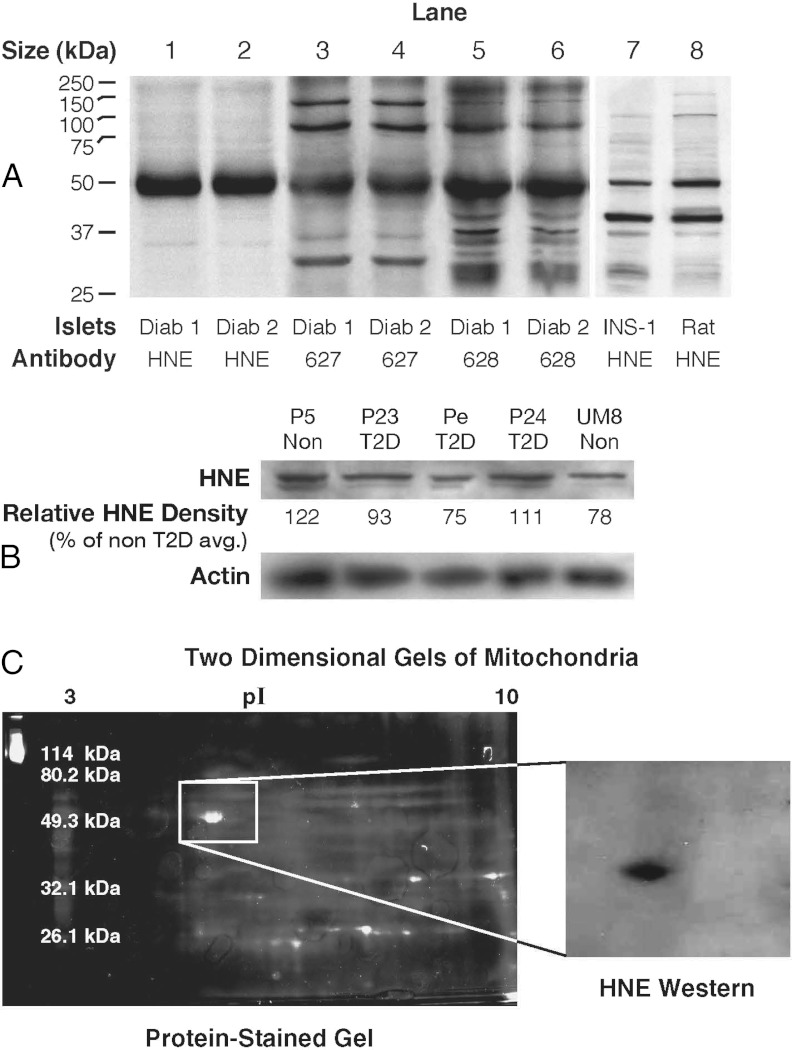
Figure 1.
Hydroxynonenal-protein adducts and other adducts in pancreatic islets from humans with type 2 diabetes and without diabetes and in rat islets and INS-1 832/13 cells. A, Immunoblots of proteins (10 μg protein per lane) from islets of humans with type 2 diabetes (Diab) from Sweden or from rats or INS-1 832/13 cells were probed with antibodies raised against 4-hydroxynonenal (HNE) by L.I.S. or 3-hydroxyoctanoic acid plus nanoic acid (627) and 4-hydroxynonenal plus nanoic acid lactone (628) raised by M.J.M. B, A different antihydroxynonenal antibody (from Chemicon) was used to analyze islets from human donors with type 2 diabetes (T2D) and nondiabetic donors (Non) from the United States. This blot (15 μg protein per lane) is representative of 4 similar blots in which islets from the same human donors with type 2 diabetes and different human donors without diabetes were analyzed. The relative density of the HNE bands are expressed as the average density of the protein bands of the islets from 2 nondiabetic donors set at 100%. The density of the 52-kDa band was not consistently darker in islets from either type of donor as judged from densitometry of the HNE band (as shown in B; also see Results). A blot of β-actin is shown as a control for loading of protein. C, Two-dimensional gel electrophoresis of the 52-kDa HNE-bonded protein from rat kidney mitochondria. The protein was partially purified from rat kidney mitochondrial proteins and then applied to 2 companion 2-dimensional electrophoresis gels that were run in parallel. The gel shown on the right was analyzed by immunoblotting with the anti-HNE antibody; the gel shown on the left was stained for protein, and the protein spot in this gel that corresponded to the anti-HNE–stained protein in the immunoblot was excised and subjected to nano-LC-MS/MS and MALDI-TOF fingerprint analysis to identify the HNE protein adduct.