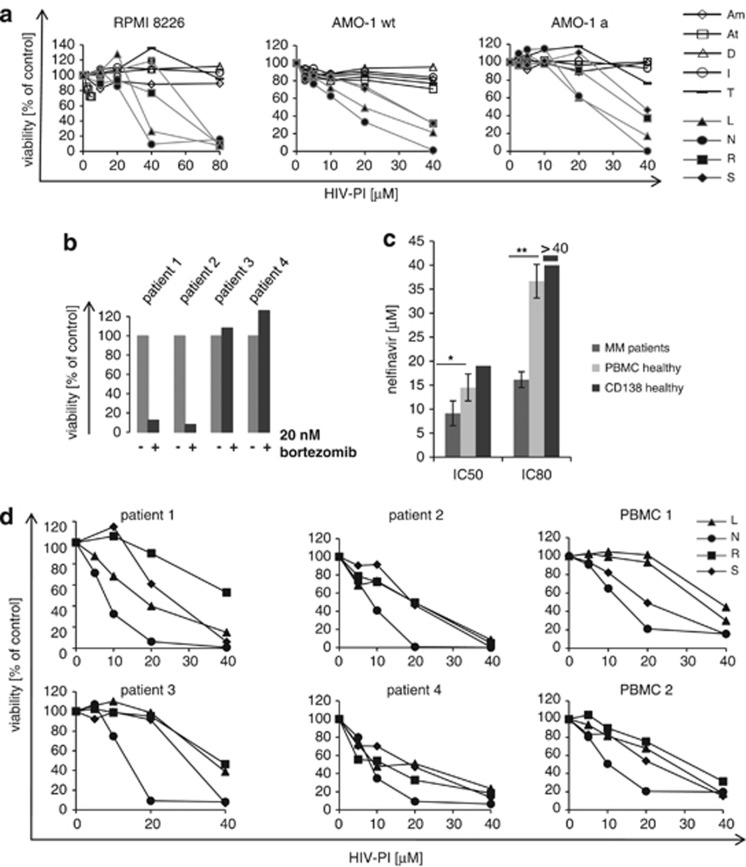
Figure 1.
Cytotoxic activity of different HIV-PI on myeloma cell lines and primary cells. (a) Myeloma cell lines (RPMI8226, AMO-1), as well as bortezomib-resistant myeloma cells (AMO-1a, in comparison with their bortezomib-sensitive wild-type (wt) parental cell lines AMO-1), were incubated with increasing concentrations of all nine approved HIV-PI (Am, amprenavir; At, atazanavir; D, darunavir; I, indinavir; T, tipranavir; L, lopinavir; N, nelfinavir; R, ritonavir; S, saquinavir) and cell viability determined by dimethylthiazol-diphenyltetrazole test. (b) Myeloma cells isolated from patients that had failed prior bortezomib-containing therapy were challenged with bortezomib 20 nℳ in vitro. Cell viability was assessed by dimethylthiazol-diphenyltetrazole test. (c) Primary myeloma cells from patients (n=4), as well as normal PBMC (n=4), as well as CD138 plasma cells from a healthy stem cell donor were incubated with increasing concentrations of nelfinavir and the IC50 and IC80 were assessed by dimethylthiazol-diphenyltetrazole test. * indicates statistically significant differences between the primary myeloma cells and PBMC from healthy donors, as assessed by student's t-test. (d) Primary myeloma cell samples characterized above and PBMC were incubated with increasing concentrations of lopinavir (L), nelfinavir (N), ritonavir (R) and saquinavir (S), and cell viability was measured by dimethylthiazol-diphenyltetrazole test.