Abstract
Social learning varies among primate species. Macaques only copy the product of observed actions, or emulate, while humans and chimpanzees also copy the process, or imitate. In humans, imitation is linked to the mirror system. Here we compare mirror system connectivity across these species using diffusion tensor imaging. In macaques and chimpanzees, the preponderance of this circuitry consists of frontal–temporal connections via the extreme/external capsules. In contrast, humans have more substantial temporal–parietal and frontal–parietal connections via the middle/inferior longitudinal fasciculi and the third branch of the superior longitudinal fasciculus. In chimpanzees and humans, but not in macaques, this circuitry includes connections with inferior temporal cortex. In humans alone, connections with superior parietal cortex were also detected. We suggest a model linking species differences in mirror system connectivity and responsivity with species differences in behavior, including adaptations for imitation and social learning of tool use.
Keywords: diffusion tensor imaging, evolution, imitation, mirror system, social learning
Introduction
Humans are experts at social learning. This is an essential building block for culture, as a behavior must be passed between individuals with high fidelity for culture to exist (Richerson and Boyd 2005). Social learning abilities vary along a continuum in primates (de Waal and Ferrari 2009; Tennie et al. 2009). While many nonhuman species are capable of observational learning, and some also have culture, in no species do these traits approach humans in degree or complexity. On a general level, the body of research on social learning has revealed a gradient in the product-oriented versus process-oriented nature of social learning from macaques to chimpanzees to humans (Tennie et al. 2009), although research continues on the specific mechanisms and processes involved (e.g. stimulus enhancement and observational conditioning). It is generally agreed that macaque monkeys do not imitate or reproduce both the physical end results of an observed action and the specific movements used to achieve it (Visalberghi and Fragazy 2002). Rather, macaques emulate or reproduce the end results of observed actions [e.g. stone handling (Huffman 1984) and potato washing (Kawamura 1959)]. Chimpanzees generally emulate rather than imitate, but some studies have reported limited process-copying in specific conditions, such as when the cause–effect relationship between an action's movements and their end result is not perceptible (Horner and Whiten 2005) or when enculturated chimpanzees are specifically trained and rewarded to “do as I do” (Tomasello et al. 1993; Custance et al. 1995). Humans have a stronger bias towards imitation, extending to “over-imitation,” or reproducing movements in an observed action that do not contribute to reaching the action's end result (Whiten et al. 2009). Another important distinction is that macaques do not acquire the tool use through social learning, while humans do; chimpanzees do so less proficiently and may rely more on individual learning for tool use acquisition (Biro et al. 2003; Tennie et al. 2009).
In humans, imitation is proposed to involve the mirror system, which includes the pars opercularis of the inferior frontal gyrus in inferior frontal cortex and the supramarginal gyrus in inferior parietal cortex. These regions are homologous to macaque regions that contain mirror neurons (ventral premotor area F5c and inferior parietal area PF), and like macaque mirror neurons, human mirror regions are active during both the observation and the execution of similar actions (Rizzolatti and Craighero 2004). The superior temporal sulcus does not have mirror properties, but it is considered an important input to the mirror system in both macaques and humans. This region's processing of biological motion (Puce and Perrett 2003) is thought to serve as the perceptual input to the frontal and parietal mirror regions (Rizzolatti and Craighero 2004). Functional neuroimaging studies on human imitation have identified activation in the inferior frontal gyrus, inferior parietal cortex, superior temporal sulcus, ventral premotor cortex, dorsal premotor cortex, and superior parietal cortex (Iacoboni et al. 1999, 2001; Molenberghs et al. 2009).
The existence of mirror systems in both macaques and humans raises a critical question: If the human mirror system supports imitation, and macaques have mirror neurons, then why don't macaques imitate? Or, on a more global level, how are neural systems for action observation–execution matching different across species? An answer to this question would have broad relevance for evolutionary social neuroscience, because neural systems for self-other matching are proposed to support not only imitation but also other social cognitive processes, such as emotional contagion and empathy (Preston and de Waal 2002).
One clue to an answer may come from an already-identified difference between the human and macaque mirror systems. Macaque mirror neurons do not respond to intransitive actions (those that lack an object, such as a mimed grasping movement) (Rizzolatti et al. 1996); in contrast, human mirror regions do (Buccino et al. 2001). This functional difference implies an underlying anatomical difference. We hypothesize that at least 1 such underlying difference could concern the organization of connections within the distributed mirror system network. Because each node of this network performs a different type of information processing, species differences in the connectivity between these nodes could produce species differences in which aspects of observed actions are “mirrored” onto the observer's own motor system, and thus copied.
Here we present diffusion tensor imaging evidence for differences in anatomical connectivity between the regions of the mirror system in macaques, chimpanzees, and humans, which parallel their differences in observational learning abilities. The putative chimpanzee mirror system has not previously been studied (its functional responses are the topic of an upcoming publication). Chimpanzees represent a telling comparison in this question, because they are humans’ closest phylogenic relatives and have social learning capacities intermediate to macaques and humans. We also propose a model linking species differences in observational learning behavior, functional brain responses, and connectivity.
Materials and Methods
Subjects
Subjects included are 1 postmortem rhesus macaque (Macaca mulatta, female, age 11 years, perfused with formalin and scanned immediately after death); a set of 5 in vivo rhesus macaques (M. mulatta, 3 females, all age 6 years); 1 postmortem chimpanzee (Pan troglodytes, female, age 28 years, scanned 14 h after death); a set of 5 in vivo chimpanzees (P. troglodytes, 5 females, mean age 14.8 years); and a set of 30 in vivo humans (Homo sapiens, male, mean age 20.2 years), all right-handed, with no history of psychiatric illness. In vivo macaque and chimpanzee subjects were scanned under anesthesia. Postmortem brains were fixed with formalin. Procedures complied with the IRB and IACUC regulations of Emory University. The 60-direction diffusion tensor imaging scans were acquired for each subject. T1-weighted structural magnetic resonance imaging scans were also acquired for in vivo subjects; B0 images were used as structural images for postmortem subjects.
Image Acquisition and Scan Parameters
Details for each subject group's scans are listed in Supplementary Table S1.
Structural Templates
We generated nonlinear T1 macaque and chimpanzee templates using FSL (http://www.fmrib.ox.ac.uk/fsl/). First, all subjects’ images were rigidly rotated into AC–PC position. The images then underwent brain extraction, bias correction, noise reduction, and contrast enhancement. Next, the images were registered to pre-existing linear templates (Rilling et al. 2007; Parr et al. 2009) using affine registration. The linearly aligned images were then summed and averaged to produce a study-specific linear template. Each subject's scan was then nonlinearly aligned to this initial linear template. Finally, the nonlinearly aligned images were summed and averaged to produce nonlinear templates.
Region of Interest Definition
Regions of interest (ROIs) were used to seed control and mirror system tractography analyses and were drawn manually and bilaterally based on published macaque (Paxinos et al. 2000) and chimpanzee (Bailey et al. 1950) maps and the human atlases implemented in FSL. Each panel in Fig. 1 shows the ROI used for that analysis. For the geniculostriate control tractography, ROIs were placed in coronal sections of the optic chiasm and occipital white matter. For the corticospinal control tractography, ROIs were placed in axial sections of the internal capsule and white matter deep to sensorimotor cortex. For the mirror system analyses, macaque ROIs were placed in areas F5c and PF/PFG; chimpanzee ROIs were placed in areas FCBm (BA 44) and PF/PFG; and human ROIs were placed in the pars opercularis of the inferior frontal gyrus (BA44) and the supramarginal gyrus (BA40). For all subjects, the superior temporal sulcus included both the dorsal and ventral banks and the fundus, extending along the entire extent of the sulcus. The inferior temporal cortex ROIs included all cortex ventral to the superior temporal sulcus ROI, terminating at the border with the parahippocampal gyrus. Thus this ROI included the inferior temporal and fusiform gyri in macaques, and the middle temporal, inferior temporal, and fusiform gyri in chimpanzees and humans. For postmortem subjects, ROIs were placed directly in the diffusion space, because only 1 postmortem subject for each species was used. For in vivo subjects, ROIs were placed on the template brain and then registered to each subject's diffusion space. We then created expanded diffusion space white matter skeletons for each in vivo subject and used these to mask ROIs. This ensured that all tractography streamlines would be started in gray matter, to avoid picking up tracts that might pass under a cortical area but not into it.
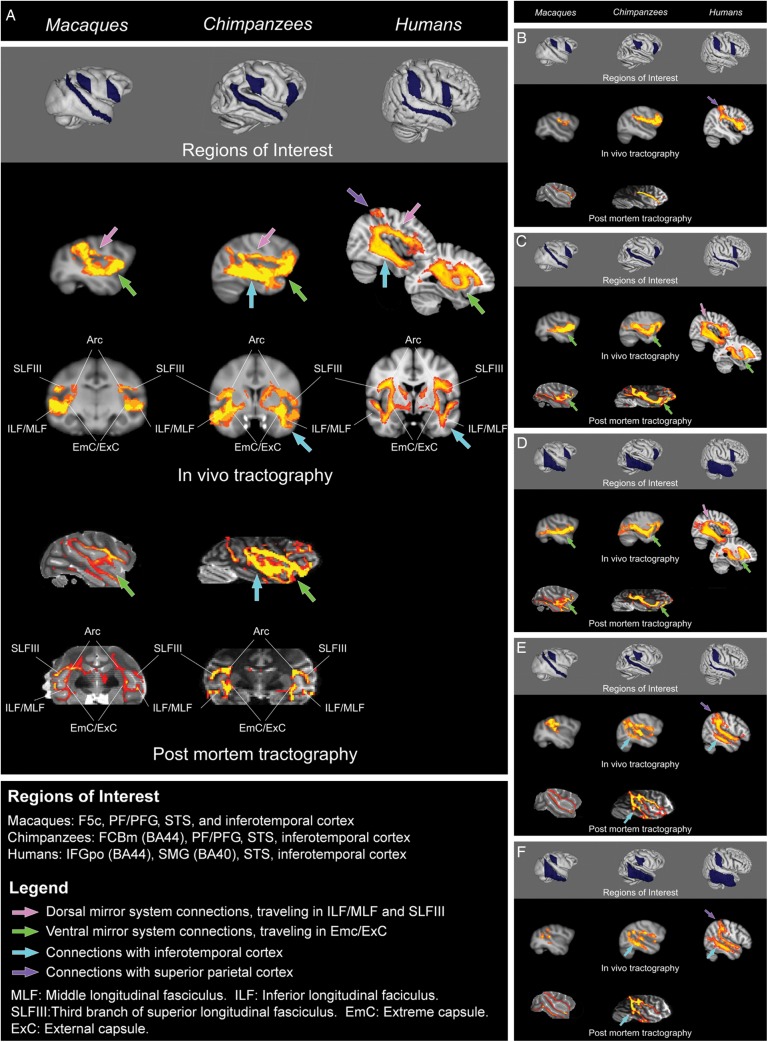
Figure 1.
(A) The mirror system as a whole: Connections between frontal mirror region, parietal mirror region, and superior temporal sulcus. Probabilistic tractography between macaque F5, PF, and superior temporal sulcus; chimpanzee BA44, PF, and superior temporal sulcus; and human BA44, BA40, and superior temporal sulcus. Three major species differences are apparent. First, there is an increase from macaques to chimpanzees to humans in the ratio of dorsal versus ventral connections within the circuit (inferior/middle longitudinal fasciculi (ILF/MLF) and third branch of superior longitudinal fasciculus (SLFIII) versus extreme and external capsules (EmC/ExC); pink versus green arrows). Second, in humans and chimpanzees, but not in macaques, this circuit includes a robust connection to inferior and middle temporal areas associated with object and tool recognition (blue arrows). Third, in humans only, this circuit includes a connection to superior parietal cortex, a region associated with spatial attention and tool use (purple arrow). Images are shown in radiological convention (right side of image corresponds to left side of brain). See online version for color figures. (B) Connections between frontal and parietal mirror regions. Probabilistic tractography between macaque F5 and PF, chimpanzee BA44 and PF, and human BA44 and BA40. These connections follow the third branch of the superior longitudinal fasciculus in all 3 species. In humans, this tract appears more robust, and includes a connection with superior parietal cortex (purple arrow). (C) Connections between superior temporal sulcus and frontal mirror region. Probabilistic tractography between macaque F5 and superior temporal sulcus; chimpanzee BA44 and superior temporal sulcus; and human BA44 and superior temporal sulcus. In all 3 species, connections between these regions follow the extreme/external capsules and pass through more anterior regions of prefrontal cortex en route to the frontal mirror region (green arrows). In humans, a second, dorsal pathway is detected, which travels through the inferior/middle longitudinal fasciculi through inferior parietal cortex to the third branch of the superior longitudinal fasciculus (pink arrow). Connections through the arcuate fasciculus (not shown here) were also detected, but this tract travels deeper in the white matter beneath parietal cortex and does not reach parietal gray matter. (D) Connections between inferior temporal cortex and frontal mirror region. Probabilistic tractography between macaque F5 and inferior temporal cortex ROI (inferior temporal and fusiform gyri); chimpanzee BA44 and inferior temporal ROI (middle, inferior, and fusiform gyri); and human BA44 and inferior temporal ROI (middle, inferior, and fusiform gyri). In all 3 species, connections between these regions follow the extreme/external capsules and pass through more anterior regions of prefrontal cortex en route to the frontal mirror region (green arrows). In humans, a second, dorsal pathway is detected, which travels through the inferior/middle longitudinal fasciculi through inferior parietal cortex to the third branch of the superior longitudinal fasciculus (pink arrow). Connections through the arcuate fasciculus (not shown here) were also detected, but this tract travels beneath the white matter of parietal cortex and does not reach parietal gray matter. (E) Sub-connections between superior temporal sulcus and parietal mirror region. Probabilistic tractography between macaque PF and superior temporal sulcus; chimpanzee PF and superior temporal sulcus; and human BA40 and superior temporal sulcus. In all 3 species, these connections follow the middle longitudinal fasciculus, but these connections extend further in chimpanzees than macaques, and furthest in humans. Connections with inferior temporal cortex via the inferior/middle longitudinal fasciculi are also apparent in chimpanzees and humans, and are more robust in humans (blue arrows). These connections also included a projection to superior parietal cortex in humans (purple arrow). (F) Connections between inferior temporal cortex and parietal mirror region. Probabilistic tractography between macaque PF and inferior temporal ROI (inferior and fusiform gyri); chimpanzee PF and inferior temporal ROI (middle, inferior, and fusiform gyri); and human BA40 and inferior temporal ROI (middle, inferior, and fusiform gyri). These connections travel through the inferior/middle longitudinal fasciculi in all 3 species. They are quite weak in macaques, robust in chimpanzees, and most robust in humans (blue arrows). In humans, a connection with superior parietal cortex is also apparent (purple arrow).
Probabilistic Tractography
FSL's software package was used to reconstruct diffusion information for all subjects. We used a probabilistic tractography algorithm designed to track through crossing fibers and into cortex (Behrens et al. 2007). This algorithm starts 25 000 “streamlines” in each voxel of the ROIs used in that analysis and tracks these streamlines through the brain, voxel by voxel, based on the orientation and size of the first and second diffusion directions in the current voxel and the surrounding voxels. We used “networks mode” tractography, which restricts the results only to those streamlines which pass through all ROIs used in a given analysis. We also used a distance correction algorithm, because connectivity probability values decrease with distance, and the distance between homologous nodes of the mirror system depends on a species’ brain size. These methods were used to examine the connectivity between the following sets of ROIs: (a) frontal–parietal–superior temporal sulcus; (b) frontal–parietal; (c) frontal–superior temporal sulcus; (d) frontal–inferior temporal cortex; (e) parietal–superior temporal sulcus; (f) parietal–inferior temporal.
Each analysis produced an image in which intensity corresponded to the probability of connectivity between all ROIs used in that analysis. However, raw values may not be directly comparable across the brains due to differences in the scan quality, voxel size, etc. Therefore, we used the following novel, conservative normalization procedure. Each image was thresholded to include only the voxels with the top 1% of the robust range of probability values for that image, where the robust range is calculated using all but the top and bottom 2% of values. For in vivo subjects, these images were then affinely registered to template space, binarized, and summed to create a composite image. In these composite images, the intensity of each voxel corresponds to the number of subjects who have a high probability of connectivity between the ROIs used in that analysis (higher than 99% of the other probability values for that image). Composite images were again thresholded to show only those voxels that were common to at least 50% of subjects. Thus, in the final composite images, all colored voxels denote areas of the brain where at least 50% of subjects had very high probability of connectivity between the ROIs for that analysis; red denotes connectivity shared by 50% of subjects and yellow denotes connectivity shared by 100% of subjects. We identified the fiber tracts carrying the observed connections (e.g. medial longitudinal fasciulus, extreme capsule, etc.) by consulting DTI atlases (Schmahmann et al. 2007; Oishi et al. 2010) alongside individual subjects’ tractography results overlaid on their color-weighted diffusion maps.
We also quantitatively compared the tractography results across species. There is no single accepted method for quantification of DTI results that relates the differences in streamline counts to the differences in actual axonal connections, especially when comparing across species. Therefore, we used the following conservative normalization procedure. Streamline counts were corrected for distance to reduce the confound of varying brain sizes. Streamline counts were also normalized by the number of voxels in the seed ROIs, since larger seed ROIs initiate more streamlines. Finally, we used the geniculostriate tract as a control pathway to normalize streamline counts in the mirror system across brains. Distance- and ROI-size-corrected streamline counts for our geniculostriate tracts also did not differ significantly between species (independent samples Kruskall–Wallis test, P = 0.377). Therefore, we divided all streamline counts for mirror system connections by that subject's geniculostriate streamline total. Thus quantitative comparisons between brains are controlled for individual and species differences in the brain size, ROI size, and scan quality. For between-species comparisons, nonparametric statistics were used since sample sizes differed and normality tests failed. For within-species comparison, normality assumptions held and parametric statistics were used.
Results
Control Tractography
To assess the reliability of our method and make certain that our results would not be confounded by variations in scan parameters or image resolution between species (Supplementary Table S1), we performed control tractography in a pathway that is unlikely to differ substantially between species, the geniculostriate pathway. Statistical comparisons revealed no significant difference in streamline numbers across species (Table 1, item A). Comparison of tractography images also revealed a qualitative similarity across species in both the geniculostriate pathway and an additional control pathway, the corticospinal tract (Supplementary Fig. S1). Importantly, the geniculostriate tractography results are consistent with a well-known species difference in the location of primary visual cortex. In humans, primary visual cortex is located on the medial face of occipital cortex, while, in nonhuman primates, it extends around the occipital pole to cover a large part of the lateral occipital lobe. The geniculostriate tractography reflects this, with the human pathway curving towards the medial face of the occipital lobe and the macaque and chimpanzee pathways terminating at the occipital poles. Thus our methods can detect species differences in features known to vary across species, but do not produce species differences in features known to be similar across species. This indicates that our analysis avoids both false-positive and false-negative results. Because the differences in spatial resolution and other scanning parameters did not produce tractography differences in these control tracts, differences in scanning procedures are unlikely to result in tractography differences in mirror system connections.
Table 1.
Quantification and statistical tests
| Question | Statistical test | Dependent variable | P value | Conclusion |
|---|---|---|---|---|
| (A) Do scan qualities differ between species? | Independent samples Kruskall–Wallis test | Number of streamlines in geniculostriate tract | 0.377 | No species differences in geniculostriate streamlines, which suggests no species differences in scan quality |
| (B) Does the overall amount of mirror system connectivity differ between species? | Independent samples Kruskall–Wallis test | Number of streamlines connecting IFG to SMG | 0.000 | Streamlines differ across species in each mirror system sub-connection |
| Number of streamlines connecting IFG to STS | 0.000 | |||
| Number of streamlines connecting SMG to STS | 0.000 | |||
| Number of streamlines connecting IFG to IT | 0.001 | |||
| Number of streamlines connecting SMG to IT | 0.000 | |||
| Step-down Mann–Whitney U test (humans vs. chimpanzees) | Number of streamlines connecting IFG to SMG | 0.001 | Humans have more streamlines in each mirror system sub-connection than chimpanzees | |
| Number of streamlines connecting IFG to STS | 0.008 | |||
| Number of streamlines connecting SMG to STS | 0.000 | |||
| Number of streamlines connecting IFG to IT | 0.040 | |||
| Number of streamlines connecting SMG to IT | 0.000 | |||
| Step-down Mann–Whitney U test (humans vs. macaques) | Number of streamlines connecting IFG to SMG | 0.000 | Humans have more streamlines in each mirror system sub-connection than macaques | |
| Number of streamlines connecting IFG to STS | 0.001 | |||
| Number of streamlines connecting SMG to STS | 0.000 | |||
| Number of streamlines connecting IFG to IT | 0.000 | |||
| Number of streamlines connecting SMG to IT | 0.000 | |||
| Step-down Mann–Whitney U test (chimpanzees vs. macaques) | No comparisons with P < 0.05 | Macaques and chimpanzees do not differ in streamlines in any mirror system sub-connection | ||
| (C) Are there between-species differences in the proportional allocation of mirror system connections between nodes? | Mann–Whitney U test (humans vs. chimpanzees) | Ratio of mirror system streamlines connecting to IFG | 0.036 | Humans have a greater proportion of mirror system streamlines devoted to the IFG node than chimpanzees |
| Mann–Whitney U test (humans vs. macaques) | Ratio of mirror system streamlines connecting to STS | 0.018 | Humans have a greater proportion of mirror system streamlines devoted to IT than macaques. Macaques have a greater proportion devoted to STS | |
| Ratio of mirror system streamlines connecting to IT | 0.000 | |||
| Mann–Whitney U test (chimpanzees vs. macaques) | No comparisons with P < 0.05 | The proportion of mirror system streamlines devoted to any node does not differ between macaques and chimpanzees | ||
| (D) Are there between-species differences in the proportional allocation of connectivity from individual mirror system ROIs to others? | Mann–Whitney U test (humans vs. chimpanzees) | Ratio of SMG streamlines connecting to IT | 0.001 | Compared with chimpanzees, humans have a greater proportion of SMG streamlines devoted to IT, a lesser proportion of STS streamlines devoted to IFG, a greater proportion of STS streamlines devoted to SMG, a lesser proportion of IT streamlines devoted to IFG, and a greater proportion of IT streamlines devoted to SMG |
| Ratio of STS streamlines connecting to IFG | 0.046 | |||
| Ratio of STS streamlines connecting to SMG | 0.046 | |||
| Ratio of IT streamlines connecting to IFG | 0.024 | |||
| Ratio of IT streamlines connecting to SMG | 0.024 | |||
| Mann–Whitney U test (humans vs. macaques) | Ratio of IT streamlines connecting to IFG | 0.001 | Compared with macaques, humans have a greater proportion of IFG streamlines connecting to IT | |
| Mann–Whitney U test (chimpanzees vs. macaques) | Ratio of IFG streamlines connecting to IT | 0.028 | Compared with macaques, chimpanzees have a greater proportion of IFG streamlines devoted to IT, a greater proportion of IT streamlines devoted to IFG, and a lesser proportion of IT streamlines devoted to SMG | |
| Ratio of IT streamlines connecting to IFG | 0.047 | |||
| Ratio of IT streamlines connecting to SMG | 0.047 | |||
| (E) Within a particular species, how are total mirror system connections allocated between nodes? | Repeated measures ANOVA with step-down T-tests (humans) | Ratio of mirror system streamlines that connect to SMG > ratio of mirror system streamlines that connect to STS | 0.014 | In humans, more mirror system streamlines are connected to SMG than STS or IT |
| Ratio of mirror system streamlines that connect to SMG > ratio of mirror system streamlines that connect to IT | 0.026 | |||
| Repeated measures ANOVA with step-down T-tests (chimpanzees) | No comparisons with P < 0.05 | In chimpanzees, there is no significant difference in mirror system streamline distributed between nodes | ||
| Repeated measures ANOVA with step-down T-tests (macaques) | Ratio of mirror system streamlines that connect to STS > ratio of mirror system streamlines that connect to F5 | 0.001 | In macaques, more mirror system streamlines are devoted to STS than to F5 or IT | |
| Ratio of mirror system streamlines that connect to STS > ratio of mirror system streamlines that connect to IT | 0.004 | |||
Only significant results are listed. All streamline counts were corrected for distance and ROI size. All mirror system streamline counts were normalized by that brain's geniculostriate streamline count.
It is important to note that our method is able to track connections across synapses (e.g. from optic chiasm to lateral geniculate nucleus to primary visual cortex). In the tractography images presented here, each colored voxel shares above-threshold connectivity, although not necessarily monosynaptically, with every seed ROI used in that analysis (see Materials and Methods). Thus, these analyses investigate the connectivity of distributed, semi-discrete networks, on a more global level than is typical of studies using injected tracers.
Mirror System Tractography
We compared mirror system connectivity across species both qualitatively and quantitatively. Qualitative results are presented first and are summarized in Figure 1. Readers are encouraged to refer to the Supplementary Material for extensive larger, additional views of tractography.
Qualitative Results
The Mirror System as a Whole: Connections Between the Frontal Mirror Region, Parietal Mirror Region, and Superior Temporal Sulcus
First, we simultaneously seeded each species’ frontal mirror region, parietal mirror region, and superior temporal sulcus. This “big picture” analysis allowed us to compare across species the connections within the mirror system as a whole (Fig. 1A; Supplementary Fig. S2A–C). Qualitative comparisons are based on tracts’ spatial extent and intensity (indicated by the color map representing a number of subjects sharing overlapping connections). We identified 3 major species differences. First, there was a variation across species in the relative size of this circuit's dorsal versus ventral components (inferior/middle longitudinal fasciculi, third branch of superior longitudinal fasciculus, and arcuate fasciculus versus extreme/external capsules; pink versus green arrows in Fig. 1A). In macaques, the ventral connection between the frontal and superior temporal nodes was much larger than the dorsal connection between the frontal and parietal nodes; in chimpanzees, the discrepancy was smaller and, in humans, these connections were more nearly equal. Second, in humans and chimpanzees, but not in macaques, this connectivity analysis yielded a projection to inferior temporal object processing regions (blue arrows in Fig. 1A). Third, in humans, but not in chimpanzees or macaques, this analysis yielded a considerable projection to superior parietal cortex (purple arrow in Fig. 1A).
To more specifically investigate which connections accounted for these species differences in anatomy and how they might relate to species differences in social learning behavior, we then separately investigated the connectivity between individual pairs of regions.
Connections Between the Frontal and Parietal Mirror Regions
In all 3 species, connections between the frontal and parietal mirror regions followed the third branch of the inferior longitudinal fasciculus (Fig. 1B; Supplementary Fig. S3A–C). This pathway appeared to be similar across species. However, in humans, these connections included a sizeable projection into superior parietal cortex (purple arrow in Fig. 1B) that was not present in macaques or chimpanzees.
Connections Between the Superior Temporal Sulcus and the Frontal Mirror Region
We found connections between the superior temporal sulcus and the frontal mirror region via similar ventral route in all 3 species (Fig. 1C; Supplementary Fig. S4A–C). These connections course through the extreme/external capsules (green arrows in Fig. 1C). Our results indicate that superior temporal sulcus in macaques is connected with the ventral frontal cortex, a region that includes area 45 as well as the frontal mirror region. As macaque tract-tracing studies do not identify direct connections between F5 and temporal cortex (Petrides and Pandya 2009), our results probably reflect multi-synaptic connections between superior temporal sulcus and area 45, which in turn connects to F5, the frontal mirror region. In humans, but not in chimpanzees or macaques, this analysis also detected a second, dorsal pathway (pink arrow in Fig. 1C). One component of this pathway passed through the parietal opercular white matter directly adjacent to the anterior supramarginal gyrus. These connections travel through the inferior/middle longitudinal fasciculi to the third branch of the superior longitudinal fasciculus. Connections through the arcuate fasciculus were also detected, but this tract travels deeper in the white matter beneath parietal cortex and does not reach parietal gray matter (Rilling et al. 2008).
Connections Between Inferior Temporal Cortex and the Frontal Mirror Region
Tractography between inferior temporal cortex and the frontal mirror region yielded similar results to tractography between superior temporal sulcus and the frontal mirror region (Fig. 1D; Supplementary Fig. S5A–C). All 3 species showed a ventral connection via the extreme/external capsules, which reached more anterior frontal regions en route to the frontal mirror region (green arrows in Fig. 1D). However, in humans, but not in chimpanzees or macaques, this analysis also yielded a second, dorsal pathway which passed through the parietal opercular white matter directly adjacent to the anterior supramarginal gyrus (pink arrow in Fig. 1D). These connections followed the inferior/middle longitudinal fasciculi and third branch of the superior longitudinal fasciculus. Connections through the arcuate fasciculus were also detected, but this tract travels deeper in the white matter beneath parietal cortex and does not reach parietal gray matter (Rilling et al. 2008).
Connections Between the Superior Temporal Sulcus and Parietal Mirror Region
In all 3 species, the superior temporal sulcus and parietal mirror region were linked by the inferior/middle longitudinal fasciculi (Fig. 1E; Supplementary Fig. S6A–C). However, this pathway showed 3 major species differences. First, the anterior extent of this pathway into the temporal lobe varied across species, being smallest in macaques, intermediate in chimpanzees, and greatest in humans. Second, in humans and chimpanzees, but not in macaques, this analysis also detected connections to the middle and inferior temporal gyri; these connections appeared more robust in humans than that in chimpanzees (blue arrows in Fig. 1E). Third, in humans, but not in chimpanzees or macaques, this analysis additionally detected a sizeable connection to superior parietal cortex (purple arrow in Fig. 1E).
Connections Between Inferior Temporal Cortex and the Parietal Mirror Region
Inferior temporal cortex and the parietal mirror region were connected by tracts similar to those connecting the superior temporal sulcus and the parietal mirror region (Fig. 1F; Supplementary Fig. S7A–C). They were very sparse in macaques, were stronger and extended more rostrally into the temporal lobes in chimpanzees, and were strongest and extended most rostrally in humans (blue arrows in Fig. 1F). These connections follow the inferior/middle longitudinal fasciculi. Additionally, in humans only, this analysis yielded a sizeable connection with superior parietal cortex (purple arrow in Fig. 1F).
Quantitative Results
We performed several statistical analyses on mirror system connectivity using streamline count in seeds to target mode probabilistic tractography as a dependent variable. A previous study used a similar approach in macaques and humans (Croxson et al. 2005). Because streamline counts vary between in vivo and postmortem datasets, we used only in vivo datasets for our quantitative analyses. We controlled for the brain size, ROI size, and differences in scan quality. We first quantitatively compared the number of streamlines in each sub-connection across species (Table 1, item B). For each sub-connection, humans had significantly more streamlines than either chimpanzees or macaques. Chimpanzees and macaques did not differ significantly from each other.
We also wondered whether 1 species’ mirror system might have relatively more or fewer streamlines connecting to a particular node. Therefore, we compared the ratio of total mirror system streamlines that reached each ROI across species (Table 1, item C). Humans have a significantly greater portion of mirror system streamlines devoted to the frontal ROI than chimpanzees, and a greater portion of streamlines devoted to the inferior temporal ROI than macaques. Macaques have a greater portion of streamlines devoted to the superior temporal sulcus than humans. These differences are qualitatively appreciable in Figure 1A (pink versus green arrows). There was no significant difference for any ROI between macaques and chimpanzees.
We also investigated whether there were species differences in the connections of particular nodes to particular other nodes. To do this, we compared the portion of each ROI's total streamlines that reached each other ROI (Table 1, item D). Compared with chimpanzees, humans have a greater proportion of supramarginal gyrus (SMG) streamlines devoted to inferotemporal cortex (IT) (appreciable in Fig. 1F), a lesser proportion of superior temporal sulcus (STS) streamlines devoted to inferior frontal gyrus (IFG) (Fig. 1C), a greater proportion of STS streamlines devoted to SMG (Fig. 1E), a lesser proportion of IT streamlines devoted to IFG (Fig. 1D), and a greater proportion of IT streamlines devoted to SMG (Fig. 1F). Compared with macaques, humans have a greater proportion of IFG streamlines connecting to IT (Fig. 1D). Compared with macaques, chimpanzees have a greater proportion of IFG streamlines devoted to IT (Fig. 1D), a greater proportion of IT streamlines devoted to IFG (Fig. 1D), and a lesser proportion of IT streamlines devoted to SMG (Fig. 1F).
Finally, we investigated the quantitative distribution of streamlines within each species, to determine which node(s) accounted for most mirror system connectivity within each species. In humans, significantly more streamlines were connected to the parietal ROI than to either the superior temporal or the inferior temporal ROIs. In chimpanzees, there were no significant differences between nodes, indicating that all have about equal numbers of streamlines. In macaques, significantly more streamlines were connected to the superior temporal sulcus ROI than to either the frontal or the inferior temporal ROIs. These differences are best appreciated in Figure 1A.
Discussion
This is the first analysis enabling direct, cross-species comparison of the organization of mirror system circuitry in macaques, chimpanzees, and humans. Following de Waal and Ferrari's (2009) theoretical approach, it provides mechanistic information that can inform a bottom-up perspective on the evolution of social learning, illustrating how biological substrates of behavior can vary in continua across species. Our results for the different species are consistent with tract-tracing and diffusion tensor imaging studies in macaques, and with diffusion tensor imaging studies in chimpanzees and humans (Petrides and Pandya 1984; Croxson et al. 2005; Makris et al. 2005; Rushworth et al. 2006; Schmahmann et al. 2007; Frey et al. 2008; Glasser and Rilling 2008; Rilling et al. 2008; Makris and Pandya 2009; Makris et al. 2009; Petrides and Pandya 2009; Ramayya et al. 2010). However, it is important to note potential limitations of this study. First, we are unable to examine tracts smaller than our largest voxel. Second, our algorithm tracks across synapses (Supplementary Fig. S1A), rather than identifying cell-to-cell connections at the level of tract tracing; therefore, our results must be interpreted as region-to-region connections at the level of closely related distributed networks. Third, this method does not allow investigation of non-connectivity-related anatomical differences that may contribute to behavioral differences, such as differences in cell types or receptor distributions. Fourth, questions about laterality and sex differences must await studies with larger sample sizes. Fifth, it is unclear how streamline counts align with actual axon counts even within a single brain, and there is no single widely accepted method for quantifying DTI data across species. While we have carefully normalized our cross-species comparisons, we suggest that readers take our quantitative comparisons as complementary to our qualitative results. Finally, there are multiple factors that can produce observed differences in the relative strength of pathways, such as path geometry, complexity, brain morphology, and data quality. However, we performed control analyses in the geniculostriate tract, which is likely to be quite evolutionarily conserved. This tract did not differ across species, suggesting that species differences in mirror system tracts are reliable.
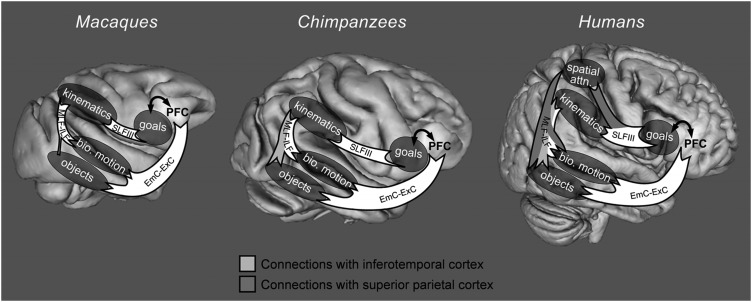
In our comparative analyses of the frontal, parietal, and temporal nodes of the mirror system, we identified 3 major species differences. Below, we consider the possible relevance of each of these differences to social learning. Our interpretation is framed around the functional roles that our ROIs may play in observation of others’ actions. We suggest that because each node of the mirror system contributes a different type of information processing, differences in their connectivity may produce observational learning circuits weighted towards different aspects of observed actions. We propose a model (Fig. 2) linking species differences in mirror system connectivity, mirror system functional responses, and social learning behavior.
Figure 2.
Product versus product in social learning: Model linking species differences in mirror system circuitry, mirror system functional responses, and social learning behavior. In macaques and chimpanzees, temporal–frontal connections via the extreme/external capsules outweigh temporal–parietal and parietal–frontal connections via the inferior/middle longitudinal fasciculi and the third branch of the superior longitudinal fasciculus. This may produce a circuit configured to mirror the product or goals of observed actions, rather than the process or kinematics, resulting in a bias toward emulation. In humans, there is a more even balance between temporal–frontal connections via the extreme/external capsules outweigh temporal–parietal and parietal–frontal connections via the inferior/middle longitudinal fasciculi and the third branch of the superior longitudinal fasciculus. This may produce a circuit that is better configured to mirror the process or kinematics of observed actions, resulting in a bias toward imitation. Additionally, chimpanzees and humans, but not macaques, have a substantial connection between the parietal mirror region and object- and tool-recognition regions in middle and inferior temporal cortex (light gray connections); this adaptation may underlie the social learning of the tool use. Finally, in humans alone, the mirror system includes a projection to superior parietal cortex (dark gray connections), an adaptation that may support spatial attention to the kinematics of others’ actions, particularly during the tool use. MLF-ILF: Middle longitudinal fasciulus and inferior longitudinal fasciculus; EmC–ExC: Extreme capsule and external capsule; SLFIII: Third branch of the superior longitudinal fasciculus; PFC: Prefrontal regions anterior to the frontal mirror region, which are connected to temporal regions via EmC–ExC, and to the frontal region via cortical U-fibers (black arrows).
The first major species difference we observed was in the relative weight of the dorsal versus ventral connections within each species’ “core” imitation circuit. Qualitatively, this is indicated by the pink versus green arrows in Figure 1A,C,D, and EmCE–ExC connections versus MLF-ILF–SLFIII connections in Figure 2. In macaques, extreme/external capsule connections far outweighed connections traveling in the inferior/middle longitudinal fasciculi and the third branch of the superior longitudinal fasciculus. In chimpanzees, this discrepancy was less pronounced. In humans, extreme/external capsule connections are relatively smaller. Quantitatively, this is reflected in the statistical tests in Table 1, item E. The most-connected node of the human mirror system is the SMG ROI; there is no significant connectivity difference in chimpanzee mirror system nodes; and the most connected node of the macaque mirror system is the STS ROI.
The ventral extreme/external capsule connections offer a route of information transfer between temporal areas which process sensory input about others’ actions (e.g. biological motion perception in superior temporal sulcus (Puce and Perrett 2003) and objects and tool recognition in inferior temporal cortex (Beauchamp and Martin 2007) and frontal areas which process higher level action goals or intentions (Johnson-Frey et al. 2003; Goldenberg 2009). Thus these ventral connections may be useful for extracting mainly the physical end result and/or goal or intention of observed actions. The dorsal connections through the inferior/middle longitudinal fasciculi and the third branch of the inferior longitudinal fasciculus link temporal sensory areas and frontal areas, respectively, with inferior parietal cortex, which is involved in the spatial mapping of movement (Johnson-Frey et al. 2003; Goldenberg 2009). These dorsal connections may be useful for extracting a finer level of kinematic detail from observed actions.
We propose that the functional relevance of this species difference may be related to biases towards emulation versus imitation, or towards copying the product versus the process of an action. Macaques’ social learning is strongly product oriented: they emulate but do not imitate. Following Lyons et al. (Lyons et al. 2006), we suggest that the macaque mirror system is mainly tuned to environmental effects of observed actions—that it “mirrors” the ends of observed actions much more than the means, or the product more than the process, due to greater temporal–frontal than temporal–parietal connections in their mirror system. Thus perhaps macaque mirror neurons do not respond to intransitive manual actions because they lack a physical end result or effect on the environment: there is nothing for their goal-oriented mirror system to “mirror.” Similarly, perhaps macaques do not imitate because imitation involves duplicating the process of an observed action, and their mirror systems compute mainly the product. In contrast to macaques, chimpanzees imitate under certain circumstances, but are biased towards emulation (Whiten et al. 2009). This may be related to chimpanzees’ stronger connections between superior temporal sulcus and inferior parietal cortex, which may allow more processing of the finer details of the spatial/kinematic structure of observed actions. Chimpanzee imitation is still quite limited compared with human imitation, and humans are even more process-oriented than chimpanzees, duplicating even those movements in an action that do not contribute to the action's overall end result (Horner and Whiten 2005). This may be related to our further-increased temporal–parietal connections. We suggest that the human mirror system is configured to “mirror” not only the product but also the process of observed actions, which could explain why human mirror regions respond to intransitive (non–object-oriented) actions.
It is important to note that frontal–temporal–parietal circuits overlapping with those studied here are also implicated in other complex cognitive functions, including language, gesture, and tool use (Frey 2007; Glasser and Rilling 2008; Rilling et al. 2008; Ramayya et al. 2010). All of these functions rely on social learning for cultural transmission. Several theories suggest that these functions may share a common neural substrate, which may or may not be the mirror system (Preuss 2007; Arbib et al. 2008; Frey 2008; Corballis 2009). Motor mirroring, and self-other matching more broadly, is likely to offer other evolutionary advantages besides social learning of manual actions. More research is needed to fully elucidate the shared versus separate nature of these cognitive functions and the contribution that each white matter tract makes to each function.
The second major species difference we observed was in the connections of the parietal mirror region with inferior temporal cortex, where objects and tools are recognized. Qualitatively, this is indicated by the blue arrows in Figure 1A,E,F, and the light gray connections in Figure 2. These connections were weak in macaques, stronger in chimpanzees, and strongest in humans. Quantitatively, this is supported by statistical comparisons in Table 1, item B: humans have more streamlines connecting SMG and IT than either macaques or chimpanzees. These results are consistent with macaque tract-tracing studies that report a paucity of connections from the area PF to inferior temporal object-processing regions (Zhong and Rockland 2003; Rozzi et al. 2006).
We propose that these connections may support the observational learning of tool use by linking information processing about the identities of objects with information processing about the spatial/kinematic details of others’ actions. The gradient in these connections from macaques to chimpanzees to humans mirrors the gradient in social learning of the tool use. Importantly, while wild macaques do not use tools, they do exhibit social learning of relatively spatially and kinematically unconstrained object-directed actions, such as potato washing and stone handling; we suggest that this is supported by information transfer between temporal and frontal cortex via the extreme/external capsules. Additionally, individual macaques can be trained to use tools in captivity. In such an experiment, Peeters et al. (2009) observed BOLD activation during the tool use observation in the anterior supramarginal gyrus in humans, but not in the corresponding area in either naive or tool-trained monkeys. These regions overlap with those used as parietal ROIs in this study. The authors propose that the anterior supramarginal gyrus contains a uniquely human tool use area that processes the cause–effect relationships between tools and actions. We suggest that an alternative or additional possible interpretation of these results is that the anterior supramarginal gyrus maps the kinematic details of observed actions onto the observer's own motor system, and that the lack of this mapping in macaques accounts for their lack of the tool use in the wild.
The third major species difference we observed was that connections between the frontal and parietal mirror regions extended furthest into superior parietal cortex in humans. Qualitatively, this is indicated by the purple arrows in Figure 1A,B,E,F and dark gray connections in Figure 2. These connections were not quantified since we did not have a superior parietal ROI in our analysis, but it is plainly evident that these connections are completely lacking in the macaque and chimpanzee tractography. Superior parietal cortex is associated with spatial awareness and attention (Husain and Nachev 2007); perhaps, this connection supports increased attention to or awareness of the trajectories of others’ actions through space. Interestingly, Hihara et al. (2006) report axon extension from anterior inferior parietal cortex to superior parietal cortex in tool-trained macaques. Additionally, superior parietal regions are activated when modern humans make stone tools in the style of our earliest tool-making hominin ancestors (Oldowan tools) (Stout and Chaminade 2007; Stout et al. 2008). These superior parietal regions are reached by the uniquely human tract identified in our analyses (purple arrows in Fig. 1A,B,E,F). Expansion of parietal cortex has been documented in hominin evolution (Bruner 2004), and modern human parietal cortex contains novel cortical areas (Orban et al. 2006). Thus we speculate that the type of information processing carried out in superior parietal cortex while observing another's action supports the social learning of the tool use.
Together, the species differences in mirror system connectivity identified here offer a proximate, anatomical explanation for species differences in observational learning. An ultimate explanation must be evolutionary. Of course, each species must be well adapted to the socioecological niche in which it evolved. Horner and Whiten (2005) suggest that chimpanzees primarily emulate because this is the most adaptive observational learning strategy for them. Attending mainly to the product of an observed action, while mainly ignoring the motor process used to achieve it, may allow chimpanzees to infer rules about object affordances and means-ends relationships on a broad enough level to generalize this information to a new situation. Compared with macaques, chimpanzees have alterations in the allocations of each ROI's connections that could support this: a greater proportion of IFG streamlines devoted to IT, a greater proportion of IT streamlines devoted to IFG, and a lesser proportion of IT streamlines devoted to SMG (Table 1, item D). We suggest that this reflects an adaptation that evolved after the macaque–chimpanzee phylogenic divergence that allows chimpanzees to mirror the product more than the process of observed actions.
Extending Horner and Whiten's idea (Horner and Whiten 2005), we speculate that humans have a greater propensity to imitate, extending to over-imitation, because this is the most adaptive learning style for the set of selection pressures we experienced during our evolution. Humans’ socially learned behaviors include actions that are much more kinematically constrained than those of chimpanzees and macaques—for example, consider bow hunting versus nut cracking and potato washing. Perhaps reproducing these more complex actions requires the capacity to copy not only observed actions’ end or product but also their means or process. Compared with chimpanzees, humans have alterations in the allocation of each ROI's connections that could support this: a greater proportion of SMG streamlines devoted to IT, a lesser proportion of STS streamlines devoted to IFG, a greater proportion of STS streamlines devoted to SMG, a lesser proportion of IT streamlines devoted to IFG, and a greater proportion of IT streamlines devoted to SMG (Table 1, item D). We suggest that this reflects an adaptation that emerged after the chimpanzee–human phylogenic divergence that allow humans to mirror the process more than the product of observed actions.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This project was supported by National Institutes of Health (RR-00165 to Yerkes National Primate Research Center; 5P01 AG026423-03 to T.M.P., J.K.R., and L.A.P.; F31MH086179-01 to E.E.H.; RO1 MH068791 to L.A.P.; MH58922, HD055255 and MH65046 to M.M.S.; and R01 MH084068-01A1 to J.K.R.), the James S. McDonnell Foundation (21002093 to T.M.P.), the Wenner-Gren Foundation (Dissertation Fieldwork Grant to EEH), the Emory University Research Committee, and the Center for Behavioral Neuroscience.
Supplementary Material
Notes
We would like to express appreciation for the expert assistance of the Yerkes Imaging Center, Biomedical Imaging Technology Center, and the Yerkes animal care and veterinary staff. Conflict of Interest: None declared.
References
- Arbib MA, Liebal K, Pika S. Primate vocalization, gesture, and the evolution of human language. Curr Anthropol. 2008;49:1053–1063. doi: 10.1086/593015. discussion 1063–1076 doi:10.1086/593015. [DOI] [PubMed] [Google Scholar]
- Bailey P, Bonin GV, McCulloch W. The isocortex of the chimpanzee. Urbana, IL: The University of Illinois Press; 1950. [Google Scholar]
- Beauchamp MS, Martin A. Grounding object concepts in perception and action: evidence from fMRI studies of tools. Cortex. 2007;43:461–468. doi: 10.1016/s0010-9452(08)70470-2. doi:10.1016/S0010-9452(08)70470-2. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. doi:10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro D, Inoue-Nakamura N, Tonooka R, Yamakoshi G, Sousa C, Matsuzawa T. Cultural innovation and transmission of tool use in wild chimpanzees: evidence from field experiments. Anim Cogn. 2003;6:213–223. doi: 10.1007/s10071-003-0183-x. doi:10.1007/s10071-003-0183-x. [DOI] [PubMed] [Google Scholar]
- Bruner E. Geometric morphometrics and paleoneurology: brain shape evolution in the genus Homo. J Hum Evol. 2004;47:279–303. doi: 10.1016/j.jhevol.2004.03.009. doi:10.1016/j.jhevol.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur J Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Corballis MC. The evolution of language. Ann N Y Acad Sci. 2009;1156:19–43. doi: 10.1111/j.1749-6632.2009.04423.x. doi:10.1111/j.1749-6632.2009.04423.x. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci. 2005;25:8854–8866. doi: 10.1523/JNEUROSCI.1311-05.2005. doi:10.1523/JNEUROSCI.1311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custance DM, Whiten A, Bard KA. Can young chimpanzees (Pan troglodytes) imitate arbitrary actions? Hayes and Hayes (1952) Revisited. Behavior. 1995;132:837–859. doi:10.1163/156853995X00036. [Google Scholar]
- de Waal FB, Ferrari PF. Towards a bottom-up perspective on animal and human cognition. Trends Cogn Sci. 2009;14:201–207. doi: 10.1016/j.tics.2010.03.003. doi:10.1016/j.tics.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Frey SH. What puts the how in where? Tool use and the divided visual streams hypothesis. Cortex. 2007;43:368–375. doi: 10.1016/s0010-9452(08)70462-3. doi:10.1016/S0010-9452(08)70462-3. [DOI] [PubMed] [Google Scholar]
- Frey SH. Tool use, communicative gesture and cerebral asymmetries in the modern human brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:1951–1957. doi: 10.1098/rstb.2008.0008. doi:10.1098/rstb.2008.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, Campbell JS, Pike GB, Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. J Neurosci. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. doi:10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008;18:2471–2482. doi: 10.1093/cercor/bhn011. doi:10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Goldenberg G. Apraxia and the parietal lobes. Neuropsychologia. 2009;47:1449–1459. doi: 10.1016/j.neuropsychologia.2008.07.014. doi:10.1016/j.neuropsychologia.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Hihara S, Notoya T, Tanaka M, Ichinose S, Ojima H, Obayashi S, Fujii N, Iriki A. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia. 2006;44:2636–2646. doi: 10.1016/j.neuropsychologia.2005.11.020. doi:10.1016/j.neuropsychologia.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Horner V, Whiten A. Causal knowledge and imitation/emulation switching in chimpanzees (Pan troglodytes) and children (Homo sapiens) Anim Cogn. 2005;8:164–181. doi: 10.1007/s10071-004-0239-6. doi:10.1007/s10071-004-0239-6. [DOI] [PubMed] [Google Scholar]
- Huffman M. Stone play of Macaca fuscata in Arashiyama B troop: transmission of a non-adaptive behavior. J Hum Evol. 1984;13:725–735. doi:10.1016/S0047-2484(84)80022-6. [Google Scholar]
- Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. doi:10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. doi:10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA. 2001;98:13995–13999. doi: 10.1073/pnas.241474598. doi:10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Frey SH, Maloof FR, Newman-Norlund R, Farrer C, Inati S, Grafton ST. Actions or hand-object interactions? Human inferior frontal cortex and action observation. Neuron. 2003;39:1053–1058. doi: 10.1016/s0896-6273(03)00524-5. doi:10.1016/S0896-6273(03)00524-5. [DOI] [PubMed] [Google Scholar]
- Kawamura S. The process of sub-culture propagation among Japanese macaques. Primates. 1959;2:45–60. [Google Scholar]
- Lyons DE, Santos LR, Keil FC. Reflections of other minds: how primate social cognition can inform the function of mirror neurons. Curr Opin Neurobiol. 2006;16:230–234. doi: 10.1016/j.conb.2006.03.015. doi:10.1016/j.conb.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Pandya DN. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–358. doi: 10.1007/s00429-008-0199-8. doi:10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. doi:10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, Kaiser JR, Sorg S, Kennedy DN, Pandya DN. Delineation of the middle longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2009;19:777–785. doi: 10.1093/cercor/bhn124. doi:10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenberghs P, Cunnington R, Mattingley JB. Is the mirror neuron system involved in imitation? A short review and meta-analysis. Neurosci Biobehav Rev. 2009;33:975–980. doi: 10.1016/j.neubiorev.2009.03.010. doi:10.1016/j.neubiorev.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Oishi K, Faria AV, van Zijl PCM, Mori S. MRI atlas of human white matter. Oxford: Academic Press; 2010. [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2006;44:2647–2667. doi: 10.1016/j.neuropsychologia.2005.11.001. doi:10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Parr LA, Hecht E, Barks SK, Preuss TM, Votaw JR. Face processing in the chimpanzee brain. Curr Biol. 2009;19:50–53. doi: 10.1016/j.cub.2008.11.04. doi:10.1016/j.cub.2008.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang X, Toga A. The rhesus monkey brain in stereotaxic coordinates. San Diego, CA: Harcourt Press; 2000. [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. The representation of tool use in humans and monkeys: common and uniquely human features. J Neurosci. 2009;29:11523–11539. doi: 10.1523/JNEUROSCI.2040-09.2009. doi:10.1523/JNEUROSCI.2040-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. doi:10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct parietal and temporal pathways to the homologues of Broca's area in the monkey. PLoS Biol. 2009;7:e1000170. doi: 10.1371/journal.pbio.1000170. doi:10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- Preuss TM. Evolutionary specializations of primate brain systems. In: Ravosa MJ, Dagosto M, editors. Primate origins: evolution and adaptations. New York: Springer; 2007. pp. 625–675. [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Philos Trans R Soc Lond B Biol Sci. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. doi:10.1098/rstb.2002.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramayya AG, Glasser MF, Rilling JK. A DTI investigation of neural substrates supporting tool use. Cereb Cortex. 2010;20:507–516. doi: 10.1093/cercor/bhp141. doi:10.1093/cercor/bhp141. [DOI] [PubMed] [Google Scholar]
- Richerson P, Boyd R. Not by genes alone: How culture transformed human evolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Rilling JK, Barks SK, Parr LA, Preuss TM, Faber TL, Pagnoni G, Bremner JD, Votaw JR. A comparison of resting-state brain activity in humans and chimpanzees. Proc Natl Acad Sci USA. 2007;104:17146–17151. doi: 10.1073/pnas.0705132104. doi:10.1073/pnas.0705132104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TE. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci. 2008;11:426–428. doi: 10.1038/nn2072. doi:10.1038/nn2072. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. doi:10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3:131–141. doi: 10.1016/0926-6410(95)00038-0. doi:10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rozzi S, Calzavara R, Belmalih A, Borra E, Gregoriou GG, Matelli M, Luppino G. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb Cortex. 2006;16:1389–1417. doi: 10.1093/cercor/bhj076. doi:10.1093/cercor/bhj076. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. doi:10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. doi:10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Stout D, Chaminade T. The evolutionary neuroscience of tool making. Neuropsychologia. 2007;45:1091–1100. doi: 10.1016/j.neuropsychologia.2006.09.014. doi:10.1016/j.neuropsychologia.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Stout D, Toth N, Schick K, Chaminade T. Neural correlates of Early Stone Age toolmaking: technology, language and cognition in human evolution. Philos Trans R Soc Lond B Biol Sci. 2008;363:1939–1949. doi: 10.1098/rstb.2008.0001. doi:10.1098/rstb.2008.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennie C, Call J, Tomasello M. Ratcheting up the ratchet: on the evolution of cumulative culture. Philos Trans R Soc Lond B Biol Sci. 2009;364:2405–2415. doi: 10.1098/rstb.2009.0052. doi:10.1098/rstb.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M, Savage-Rumbaugh S, Kruger AC. Imitative learning of actions on objects by children, chimpanzees, and enculturated chimpanzees. Child Dev. 1993;64:1688–1705. doi:10.2307/1131463. [PubMed] [Google Scholar]
- Visalberghi E, Fragazy D. "Do monkeys ape?" ten years after. In: Dautenhahn K, Nehaniv CL, editors. Imitation in animals and artefacts. Cambridge(MA): MIT Press; 2002. pp. 471–499. [Google Scholar]
- Whiten A, McGuigan N, Marshall-Pescini S, Hopper LM. Emulation, imitation, over-imitation and the scope of culture for child and chimpanzee. Philos Trans R Soc Lond B Biol Sci. 2009;364:2417–2428. doi: 10.1098/rstb.2009.0069. doi:10.1098/rstb.2009.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong YM, Rockland KS. Inferior parietal lobule projections to anterior inferotemporal cortex (area TE) in macaque monkey. Cereb Cortex. 2003;13:527–540. doi: 10.1093/cercor/13.5.527. doi:10.1093/cercor/13.5.527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.