Abstract
A subpopulation of men that appear cured of prostate cancer (PCa) develop bone metastases many years after prostatectomy. This observation indicates that PCa cells were present outside of the prostate at the time of prostatectomy and remained dormant. Several lines of evidence indicate that there are disseminated tumor cells (DTCs) in the bone marrow at the time of prostatectomy. DTCs parasitize the bone microenvironment, where they derive support and impact the microenvironment itself. These DTCs appear to be a heterogeneous population of PCa cells; however, some of them appear to have some aspects of a cancer stem cell (CSC) phenotype as they can develop into clinically detectable metastases. The concept of CSC is controversial; however, several markers of CSC have been identified for PCa, which may represent cells of either basal or luminal origin. These DTCs have now been shown to compete for the hematopoietic stem cell niche in bone, where they may be placed in a dormant state. Interaction with a variety of host factors, including cytokine and cells, may impact the metastatic development and progression, including the dormant state. For example, myeloid cells have been shown to impact both the premetastatic niche and established tumors. Understanding the concepts of how PCa successfully parasitizes the bone microenvironment is paramount toward identifying therapeutic candidates to prevent or diminish PCa bone metastases.
Keywords: cancer stem cell, monocytes, prostate cancer, hematopoietic stem cell, microenvironment
I. INTRODUCTION
Mr. Z. is a 63-year-old man. Seven years ago, he was diagnosed with a moderately differentiated, localized prostate cancer (PCa) when he presented for a routine physical exam, and was found to have a prostate-specific antigen (PSA) blood test of 4.1 ng/ml. Digital rectal exam revealed no abnormalities, but prostate ultrasound and biopsy revealed a Gleason 3 + 3 = 6 cancer in 4/12 biopsy cores (clinical stage T1cNxMx). Because Mr. Z. was in otherwise excellent health, he chose to undergo a radical retro-pubic prostatectomy, and his prostate was removed. All of his lymph nodes were negative for cancer. By all clinical and pathologic predictors, he was considered to be cured of his disease. One year ago, Mr. Z.’s PSA became detectable and he now has two lesions present on bone scan. He has metastatic PCa, and his disease is now incurable.
Each year, ~40,000 men who “should” have been cured of their PCa by surgery or radiation therapy present with incurable metastatic disease that will manifest itself as metastatic lesions in the bone, usually years after primary treatment. The best explanation for this is that the dissemination of tumor cells to the bone microenvironment before surgery or radiation eradicated the primary tumor. Currently, there are no effective therapies for disseminated tumor cells (DTCs) that then become clinically evident metastases. Understanding the process of cancer metastasis continues to fascinate and frustrate cancer biologists and clinicians. How circulating tumor cells (CTCs) traffic to the bone, and how DTCs become dormant and ultimately overcome dormancy to proliferate, remain open and critical questions. In this review, we will discuss potential mechanisms that attract PCa to bone and allow them to establish footholds and thrive in the bone microenvironment.
II. CIRCULATING TUMOR CELLS AND DISSEMINATED TUMOR CELLS
In 1889, Stephen Paget observed distinct metastatic patterns of 735 breast cancer patients. He found that breast cancer had a high propensity to spread to certain secondary sites, such as the marrow, while growth in other secondary sites was less common. This led him to hypothesize that metastatic cells or “seeds” must fall on “congenial soil.”1 Since that time, cancer researchers have expanded on these studies in an attempt to understand the biology of metastasis and identify biomarkers for diagnosis and prognosis as well as targets for therapeutic intervention. Primary tumors are known to interact with a diverse population of nonmalignant cells, forming a tumor ecosystem.2 An ecosystem is defined as “any unit that includes all of the organisms (the biotic community) in a given area interacting with the physical environment so that a flow of energy leads to clearly defined biotic structures and cycling of materials between living and nonliving components is an ecological system.”3 Using the ecosystem paradigm, cancer cells, growing in an organ, can be considered to be a species coexisting in a complex habitat with other host cells. Cancer cells and host cells, interacting within their habitat, create an ecosystem that exists within the larger environment of the patient. The ecological principles can be applied to understanding tumor biology and metastasis.3,4
Invasive species and their impact on local environments as well as the global ecosystem have been well documented.5–8 Invasive species begin as a native population within a community of organisms, then either migrate or are transported to a new environment/ecosystem.5 In this new environment, the invader either dies off or enters a period of time during which it establishes itself (lag period). The species then begins to spread, and disrupts the ecosystem as a whole. Successful invasive species have biologic traits that include rapid proliferative capacity, adaptation to environmental stress (phenotypic plasticity), and high tolerance to environmental heterogeneity.9–11
The life cycle of invasive species is directly analogous to the study of cancer metastasis.4–11 Cancer must grow in a primary organ, extravasate, and survive in the circulation, and then intravasate at a target organ (invasive species’ survival in transport). Cancer cells are believed to lay dormant at their metastatic site for a long period of time (lag period) before proliferating (invasive spread). Proliferation in the new site has an impact on the target organ microenvironment (ecological impact) and eventually the human host (biosphere impact).11–13 Understanding how prostate cancer cells invade the bone marrow, establish themselves, and then either lay permanently dormant or eventually proliferate and naturalize to become the dominant species in the bone marrow ecosystem is of paramount importance.
It is clear that primary clinically localized PCas shed tumor cells into the circulation.14 In a recent study of 228 men with elevated PSA who were undergoing a biopsy for suspected PCa, 29% of the biopsies were positive for PCa and 86% of these men had CTCs. In landmark studies by Vessella and colleagues, they assessed CTCs and DTCs in patients undergoing surgical treatment for their clinically localized prostate cancer.15,16 Prior to surgery, the investigators detected PSA-expressing epithelial cells in ~50% of bone marrow aspirates (DTCs) and 24% of peripheral blood samples (CTCs). Even at a median time of four months after prostatectomy, PSA-expressing cells were detected in 33% of bone marrow samples and 9% of blood samples from men without evidence of disease, suggesting a continued population of tumor cells present in the host patient. In men more than five years after prostatectomy, DTCs were detected in almost 30% of the men (four of 14 men). Two of these men had no evidence of clinical disease, and two of these men had developed disease recurrence.
This data has been corroborated by other investigators. Gao and colleagues detected PSA-expressing cells by RT-PCR PSA assays in bone marrow of 51 of 116 patients undergoing prostatectomy for treatment of presumed localized disease (44.0%).17 Twenty-five men (49%) had organ-confined disease, and 26 (51%) had non–organ-confined disease at the time of surgery. Hauntingly, detection of DTCs was not associated with age, race, grade, pretreatment PSA, clinical stage, or margin status. This suggests that the presence of DTCs was independent of every classic prognostic predictor in the field. The investigators did find, however, that the presence of DTCs was associated with disease recurrence. The two-year disease-free survival was 97% in RT-PCR negative patients versus 78% in RT-PCR positive patients (p = 0.054).
While it is yet to be proven, it is generally believed that a subset of CTCs survive the circulation and take up residence in the bone marrow as DTCs.18–20 Importantly, DTCs have been shown to express genetic heterogeneity, implying that the populations of cells that clonally expand into overt metastases is selected early in the dissemination process.(21 It is plausible that the selected DTCs have cancer stem cell (CSC)–like phenotypes, indicating that an understanding of prostate CSC may be important to identify effective therapies against PCa.
III. PROSTATE CANCER STEM CELLS
CSCs have been suggested to account for chemoresistance, radioresistance, and tumor dormancy and metastasis. The concept of CSC was introduced more than 50 years ago when it was recognized that only a small proportion of cells (0.01%–1%) in tumor isolates are clonogenic and extensively proliferative in vitro and in vivo, 22,23 indicating that these cells might represent tumor stem cells. The CSC hypothesis has recently been revitalized due to the development of novel methods for identification, purification, and characterization of normal stem cells. Although no consensus definition of a CSC exists, a general descriptor is “a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor.”24 There is sufficient complexity in the CSC field to suggest the CSC do not adhere to any rigorous definition, and instead the concept of a tumor-initiating cell (TIC) should be considered. The TIC, while similar to CSC, may not need to strictly adhere to the self-renewal properties and ability to recapitulate all tumor cell types.
A. Origin of Prostate CSCs
The origin of prostate CSCs is controversial, and in fact they may represent different tissue components of the prostate. However, the cell of origin is highly relevant for the prostate CSCs since different cells of origin may generate clinically relevant subtypes with different prognosis and outcome. In breast cancer, different tumor subtypes have been proposed to originate through transformation of different progenitors within the mammary epithelial lineage hierarchy.25 There are two possible cell-of-origin resources in PCa, namely, basal cell of origin and luminal cell of origin.
Basal cells express multiple molecules that regulate stem cell self-renewal and survival, such as p63, hTERT, and Bcl-2,26,27 and additionally express stem cell markers including CD44, CD49f, CD117, CD133, Tert, and p63.28 Thus, the prostate basal cell layer has been hypothesized to harbor stem cells.29,30 Multiple lines of evidence support this possibility. For example, basal prostate CSCs with a CD44+α2β1integrinhighCD133+ phenotype were successfully isolated from human PCa biopsies. 31 Mouse Lin−Sca-1+CD49fhigh cells correspond to a predominantly basal population, and can differentiate into luminal cells in grafts.32 Basal Lin−Sca-1+CD49fhigh cells have the capacity to form tumorlike spheroids in vitro and grafts in vivo.33 Deletion of Pten in Pten-null mice is associated with an increase in p63+ basal cell numbers and the expansion of a prostate stem/progenitor–like subpopulation and consequent tumor initiation.34 The strongest tumor-initiating fraction where <100 cells are required to initiate new tumor growth in immune-compromised mice has been confirmed to have a basal phenotype.35 A study with FACS-sorted primary cells showed that basal cells, but not luminal cells, are the cells of origin for PCa.36,37 Basal cells from primary benign human prostate tissue can initiate prostate cancer in immunodeficient mice.36 The recurrent gene fusions of the 5’ untranslated region of TMPRSS2 to ERG or ETV1 in PCa tissues with outlier expression was identified by Tomlins et al.,38 TMPRSS2–ERG is expressed in CD44+α2β1integrinhighCD133+ cells from prostate tumors,39 which supports the hypothesis that the cell of origin of PCa is a basal stem cell.35 Recently, Rajasekhar et al. identified a small population of TRA-1-60+/CD151+/CD166+ TICs isolated from human prostate xenograft tumor. These stemlike sphere cells do not express AR, PSA, or CK18, and are of basal epithelial–like cell type based on the expression of E-cadherin, CK5, and SOX9, and lack of expression of markers of myoepithelial cells (smooth muscle actin), mesenchemal cells (vimentin), and neuroendocrine cells (synaptophysin). However, these sphere cells also lack detectable expression of basal cell enriched p63 and its polarity associated zonula occludens-1(ZO-1).40 Thus, there is a strong line of evidence that many prostate CSCs are derived from basal cells. However, evidence has also accumulated for luminal-cell-of-origin prostate CSC.
Recent studies have provided support for the luminal cell-of-origin theory for prostate CSC. For instance, in Pten knockout mice, single pAkt+ cells in the luminal epithelial cell layer overexpressed CK8, Sca-1, Tacstd2, and Clu, whereas basal epithelial cells were always pAkt−.41 Importantly, Clu+Tacstd2+Sca-1+ progenitor cells, which are candidate TICs, were detected in the luminal epithelial cell layer of normal prostates (41). The initial hyperplastic cells were all luminal as well.41 Genetic lineage marking demonstrates that rare luminal cells that express Nkx3.1 (androgen/AR–regulated transcriptional coactivator) in the absence of testicular androgens (castration-resistant Nkx3-1–expressing cells—CARNs) are bipotential and can self-renew in vivo, and single-cell transplantation assays show that CARNs can reconstitute prostate ducts in renal grafts.42 Functional assays of Nkx3.1 mutant mice in serial prostate regeneration suggest that Nkx3.1 is required for stem cell maintenance. Furthermore, targeted deletion of Pten in CARNs leads to high-grade PIN and rapid carcinoma formation after androgen-mediated regeneration. These observations indicate that CARNs represent a new luminal stem cell population that is an efficient target for oncogenic transformation in prostate cancer.42
The origin of PCa and the cell type of origin remains controversial in part because distinct functional assays were employed. Furthermore, because PCa is a very heterogeneous disease, it is plausible that different PCas are derived from different originating cell types.
B. Putative Markers of Prostate CSCs
Prostate CSCs express a number of the same markers as prostate stem cells, such as CD44, CD133, integrins, breast cancer–resistance protein (BCRP), and Sca-1, all of which have been utilized to identify prostate CSCs or prostate stem cells.
CD44 has been proven to be a candidate marker for normal prostatic epithelium stem cell and prostate CSCs.26 CD44+ PCa cell population is enriched in tumorigenic and metastatic progenitor cells. CD44+ PCa cells are more proliferative, clonogenic, tumorigenic, and metastatic than the isogenic CD44− PCa cells.43 CD44+ PCa cells have been evaluated with a series of characteristics43: possess certain intrinsic properties of progenitor cells; colocalize with a population of intermediate label-retaining cells; express higher mRNA levels of several “stemness” genes including Oct-3/4, Bmi, β-catenin, and SMO; generate CD44− cells in vitro and in vivo. CD44+ PCa cells, which are androgen receptor (AR−), can differentiate into AR+ tumor cells. A very small percentage of CD44+ PCa cells appear to undergo asymmetric cell division in clonal analyses.43 CD44+/CD24− LNCaP cells could form prostaspheres in vitro.44 CD44+/CD24− cells form colonies in soft agar and form tumors in NOD/SCID mice when as few as 100 cells are injected.44 Interestingly, expression of CD44 is associated with cells of NE phenotype, which is of significance in therapy resistance and tumor recurrence. 45 Long-term maintained sphere-propagating DU145 cells with stemlike properties are also enriched with CD44, CD24, and integrinα2β1.46 All of the above evidence confirms that CD44 is a prospective marker for prostate CSCs.
CD133 has been proposed to be a putative surface marker in a number of tumors, as mentioned above. Collins et al. found only tumor-derived CD133+ cells were capable of self-renewal and extensive proliferation (31). CD133+ cells, enriched in the CD44+ integrinα2β1high basal population and representing ~0.75% of basal cells, were shown to possess a high in vitro proliferative potential and are able to reconstitute prostaticlike acini in ~20% recipient nude mice.47 However, the CD133− cell population also contained clonogenic cells and the prostaticlike acini were not very typical structures.47 In DU145 cells, the clones formed by CD44+ integrinα2β1highCD133+ subpopulation are remarkably different morphologically and quantitatively from those formed by integrinα2β1−/low CD133− cells, and CD133+ cells have the capacity of self-renewal, extensive differentiation potential, and high proliferative and tumorigenic potential.48 Within a series of AR+ human PCa cell lines including LAPC-4, LNCaP, and CWR22Rv1 cells, CD133+ cells are present at a low frequency, self-renew, express AR, generate phenotypically heterogeneous progeny negative for CD133, and possess an unlimited proliferative capacity.49 However, other investigators found that CD133 was only expressed in DU145 cells, except for DuCaP, LAPC-4, CWR22Rv1, LNCaP, and PC3 cells, and considered CD133 selection does not enrich for stemlike cells in PCa cell lines.50 This variance may be caused by the application of different antibodies to CD133.
ALDH is an enzyme involved in intracellular retinoic acid production.51 In prostate CSCs studies, the high expression of ALDH1A1, a member of the ALDH family, was found to be positively correlated with Gleason score and pathologic stage, and inversely associated with overall survival and cancer-specific survival of the patients, indicating ALDH1A1 could be a potential prostate CSC-related marker .52 The ALDHhi cells have greater in vitro proliferative potential than cells with low ALDH activity, and high levels of ALDH activity might be a functional marker of murine prostate stem/progenitor cells.53 Van den Hoogen et al. successfully used high ALDH activity to identify tumor-initiating PCa cells and metastasis54: ALDHhi PCa cells not only display enhanced clonogenicity and migration in vitro, but also show enhanced tumorigenicity and metastatic ability in vivo. These cells demonstrate increased metastatic ability in vivo as well.54 We have shown that ALDH activity indicates increased tumorigenicity, but not a CSC phenotype, in PCa cell lines.55 We found that ALDHhi CD44+ cells exhibit a higher proliferative, clonogenic, and metastatic capacity in vitro and demonstrate higher tumorigenicity capacity in vivo than ALDHlo CD44-cells. However, ALDHlo CD44− cells were able to develop tumors, albeit with longer latency periods.55 The conflicts among the different studies may be caused by different cell lines utilized in different groups and by the complexity and diversity of PCa cell lines. Clonally derived holoclones are thought to contain self-renewing stem cells, whereas meroclones and paraclones consist of transit amplifying cells.56 Isolation of ALDHhi PC3 cells enriches for the most primitive holoclone population.57 Therefore, ALDH activity is a promising surface marker for prostate CSCs in clinically derived tissues. Regardless of the origin and/or markers that define prostate CSC, it appears that the majority of these cells become dormant as they take up residence in the hematopoietic stem cell (HSC) niche, awaiting to be awakened by genetic instability and pressures from the bone marrow microenvironment.
IV. THE HEMATOPOIETIC STEM CELL NICHE
The process of blood cell formation by which the pluripotent HSCs give rise to all blood cells is known as “hematopoiesis.” Blood cells first arise in the yolk sac from two to three weeks’ gestation to eight weeks’ gestation. Subsequently, the fetal liver serves as the major site of hematopoiesis from five to six weeks’ gestation through six months’ gestation. Thereafter, bone marrow begins hematopoiesis at seven months’ gestation and takes over the responsibility for blood cell formation by birth. Bone marrow is a soft fatty tissue that consists of the cellular components, including fibroblasts, osteoblasts, osteoclasts, reticulocytes, endothelial cells, adipocytes, mesenchymal stem cells (MSCs), and the cells of hematopoietic origin, as well as the vascular compartment. Recent studies have revealed that nonhematopoietic cells serve as the microenvironment for HSCs in the marrow to regulate hematopoiesis.58–66 That microenvironment is referred to as the “HSC niche.”
The concept of the HSC niche was first proposed in 1978,67 because surrounding supportive cells regulate the behaviors of adult stem cells. Within the niche, HSCs are capable of maintaining their self-renewal and pluripotency. To the contrary, HSCs differentiate irreversibly into progenitor cells when they detach from the niche. Intriguingly, more recent study has suggested that the HSC niche also contributes to the development of hematologic disease,68 further suggesting that the fate of HSCs depends directly on the niche. Despite vigorous debates about the anatomical location of the HSC niche,69,70 the bone marrow HSC niche is considered to be dominated by two main cellular components, i.e., the osteoblastic niche59–62 and endothelial niche,63 while the participation of other cell types has also been described.64–66 It is suggested that the osteoblastic niche maintains the quiescent of HSCs, whereas the endothelial niche regulates the proliferation and differentiation of HSCs.69,71
In 1994, the role of human osteoblasts in the maintenance of HSCs in an ex vivo culture system was first proposed.58 About a decade later, the hypothesis that osteoblasts are a crucial component of the HSC niche was confirmed using animal models.61,62 When the number of osteoblasts was increased by genetically upregulating the receptor for parathyroid hormone (PTH) on osteoblasts, the enhancements of hematopoiesis through Notch/Jagged1 pathway were observed.61 Likewise, when the number of N-cadherin–positive osteoblasts was increased by genetic knockdown of the bone morphogenetic protein (BMP) receptor 1A, the number of HSCs was significantly increased.62 To the contrary, hematopoiesis was dramatically impaired following the decline of HSCs when the osteoblastic niche in Col2.3Δ-TK transgenic mice was selectively depleted with ganciclovir,72,73 Although many molecules are likely to be produced in the osteoblastic niche that regulate hematopoiesis either positively or negatively, recent findings suggest that HSC quiescence is regulated through thrombopoietin (THPO) and its receptor Mpl,74,75 and the Tie2/Ang-1 signaling pathways.60 Regulating hematopoiesis in the opposite direction. Osteopontin, a matrix glycoprotein expressed by osteoblasts has been shown to negatively regulate hematopoiesis by inducing HSCs into the cycles.76,77
Osteoblasts also regulate HSC homing and engraftment through the CXCR4/CXCL12 pathway as a major source of CXCL12 in the bone marrow. 59,78 HSCs can be mobilized from the osteoblastic niche by degrading CXCL12 with the granulocyte colony–stimulating factor (G-CSF)79 or by inhibiting the interaction of CXCR4/CXCL12 with a CXCR4 inhibitor AMD3100.80 Interestingly, circadian rhythm appears to be involved in CXCL12-dependent HSC homing.81,82 It has also been demonstrated CXCL12-independent mechanisms of HSC homing including annexin II (Anxa2),83 the calcium-sensing receptor,84 the guanine nucleotide–binding protein stimulatory α subunit,85 c-kit, 86 and the very late antigen-4 (VLA-4).87,88
In 2005, data convincingly emerged that there was at least a second niche that regulates HSC function. Here it was demonstrated at sinusoidal endothelial cells participate in the creation of an HSC niche.63 The basis of this finding was the ability to isolate and identify HSCs in vivo, where the majority of HSCs, isolated based on the SLAM family receptors including CD150, CD244, and CD48, colocalized to the sinusoidal endothelial cells within the bone marrow and spleen, while some HSCs were observed near the osteoblastic niche.63 In addition, the sinusoidal endothelial cells supported THPO-independent thrombopoiesis.89 However, it is difficult to delete endothelial cells in vivo to investigate the functional effects of endothelial cells on the maintenance of HSCs because endothelial cells line the inside of all blood vessels of the body. Although little has been known about the role of endothelial niche in hematopoiesis, endothelial cells appear to serve as the HSC niche, since they are the gateway between the bone marrow and the circulation.
A recent study has revealed that HSCs themselves participate in the creation of the osteoblastic HSC niche. HSCs are able to stimulate bone formation by expressing BMPs in response to hematologic stress.90 Intriguingly, erythropoietin (Epo) appears to be involved in this BMP production in HSCs via the Jak2/Stat3 signaling pathway. 91 These findings also suggest that the cross talk between HSCs and the niche is crucial for maintaining hematopoiesis. However, it is less clear if one alone constitutes a single cell type that controls hematopoiesis— more than likely the participation of multiple cell types is required. Indeed, where the niche cells located in the marrow has been continuously under debate. Although further study would be clearly needed, it is likely that osteoblasts, endothelial cells, and/or other cell types compose the HSC niche. Furthermore, it is important to know whether the multiple niches cooperate and influence each other to regulate hematopoiesis.
V. PROSTATE CANCER NICHE
The prognosis of PCa patients with distant organ metastasis remains poor. PCa frequently spread to the bone marrow.15,92 Growing evidence has suggested that the microenvironment within the bone marrow plays a crucial role in the development of metastasis.93,94 Therefore, a hypothesis worth exploring is that bone metastasis targets the microenvironment that supports hematopoiesis, since the bone marrow is the primary site of hematopoiesis. Other evidence that supports this hypothesis is that bone metastatic cancer cells disseminate in similar fashion to how HSCs home to the marrow such as the CXCR4/CXCL12 axis.95,96 In addition to the homing, both the engraftment of HSCs and the metastasis of PCa were dramatically compromised when the expression of the adhesion molecules including Anxa2 (Refs. 83 and 97) and VLA-4 (Refs. 87, 88, and 98) were suppressed. These findings strongly suggest the parallels between the mechanisms of the HSC homing to bone marrow and bone metastasis.
We have recently shown that DTCs parasitize the bone marrow HSC niche.99 In this study, the metastatic behavior of DTCs was assessed using an in vivo micrometastasis model that we recently developed.100 First, direct competition for the niche between HSCs and PCa was demonstrated. Engraftment of transplanted HSCs were diminished when the marrow was preoccupied by DTCs.99 Simultaneous injection of PCa also inhibited that ability of bone marrow transplantation to rescue the bone marrow from lethal radiation, and PCa-bearing animals showed less survival.99 Furthermore, HSCs and DTCs colocalized close to the osteoblastic niche.99
If the DTCs target the HSC niche, then it is reasonable to assume that metastasis would be altered by manipulating the size of the niche. Bone anabolic PTH treatment to expand the niche61 resulted in significantly more DTCs in the marrow,99 whereas selective ablation of the osteoblastic niche using subcutaneous implantation of thymidine kinase–transformed bone tissues73 resulted in significantly fewer DTCs in the implanted bone tissues. 99 Next, it was demonstrated that the CXCR4/CXCL12 axis contributed to the mechanisms of niche competition of HSCs and DTCs. DTCs were displaced from the marrow by degrading CXCL12 (G-CSF) and by blocking the CXCR4/CXCL12 interaction (AMD3100).99 Moreover, more DTCs were found in the marrow when HSCs were cleared from the marrow using AMD3100 prior to the PCa injections.99 Finally, it was revealed that DTCs affect the function of HSCs once they settle in the marrow niche. When DTCs were established in the marrow, less HSCs were observed in the marrow, while more hematopoietic progenitor cells (HPCs) were found.99 Interestingly, the gene expression related to the the hematopoietic stem cell phenotype was downregulated in these HSCs.99 More importantly, more HPCs were found in the blood collected from the PCa patients with bone metastasis compared to the healthy controls or patients without metastasis.99 Collectively, these findings strongly suggested that HSCs and DTCs target the same area in the marrow by sharing the same molecular axis, and that the HSC niche plays a supportive role in the PCa dissemination.
The observation that DTCs target the HSC suggests that other functions of the HSC niche could impact PCa growth in the niche. For example, in hematopoiesis, the HSC niche provides signals that regulate both (i) HSC quiescence to maintain the self-renewal and (ii) their differentiation to populate the entire hematopoietic system.59 This could account for the observation that DTCs can rest and form distant metastases years or even decades later.101–103 This critical phenomenon is referred to as “tumor dormancy.”104 It is believed that once DTCs become dormant, they acquire the ability to escape the cell death. However, to date little is known about how the DTCs become dormant in the marrow. If DTCs target the HSC niche during dissemination, then DTCs may hijack the mechanisms to regulate the function of HSCs to survive in the marrow.
It has been proposed that the microenvironmental growth arrest specific-6 (Gas6) is involved in HSCs’ fate.105 The fibroblasts supported the repopulation ability of murine hematopoiesis in in vitro culture system when the cell-associated Gas6 was genetically upregulated.105 Gas6 is known as a ligand for Axl, TYRO3 (SKY), and MER. It was revealed that the AXL expression on the HSCs plays a regulatory role in hematopoiesis. 106 Like HSCs, PCa also expresses the AXL subfamily of tyrosine kinase receptors, and their expression was enhanced when PCa bound to the osteoblastic niche through the Anxa2 and/or its receptor.107 Importantly, Gas6 produced by the niche stimulating the survival signals in the PCa including chemoresistant ability by inducing tumor dormancy through AXL.107 Consistent with this notion, the Gas6/MER interaction protects leukemia cells from chemotherapy-induced apoptosis. 108
Another interesting molecule that is involved in both HSC the quiescence and PCa dormancy is BMP. It has been demonstrated that BMP- 2, -4, and -7 treatments are able to maintain the CD34+CD38−Lin− stemlike phenotype in ex vivo culture of human cord blood HSCs.109 In addition, HSCs treated with BMP-4 were capable of engrafting into immunodeficient animals after six days of ex vivo culture.109 Likewise, BMP-7 secreted by bone marrow stromal cells is involved in the mechanisms of PCa dormancy in the marrow. 110 BMP-7 induced the cell cycle and growth arrest in PCa cells by activating p38 MAPK, and increased cell cycle inhibitor p21 and the metastasis suppressor gene NDRG1.110 Interestingly, the cell cycle and growth arrest were reversibly inhibited when BMP-7 treatment was stopped.110
Although Stephen Paget espoused his “seed and soil” hypothesis over 100 years ago,111 we are still just discovering how this manifests at the molecular and cellular levels. It has become increasingly evident that the disseminated PCas share the function of the HSC niche with HSCs to survive in the heterotopic site. Yet, how these two cell types share the pathway remains largely unexplored. Further study will therefore doubtless give better insight into the microenvironment for disseminated PCa, or the PCa niche. Moreover, targeting PCa-niche interactions could be a potential therapy for PCa bone metastasis. Multiple cells within the HSC niche may further modify how the PCa cells maintain or escape from dormancy. For example, myeloid cells represent a large component of cells found in the bone marrow, and have many properties that can impact tumor growth.
VI. MYELOID LINEAGE CELLS IN THE NICHE
A. Roles of Myeloid Cells
Myeloid progenitor cells differentiate to a variety of cells including neutrophils, basophils, eosinophils, monocyte/macrophages, dendritic cells, osteoclasts, and megakaryocytes.112 Myeloid cells in the tumor microenvironment are proven supporters of tumorigenicity, and are emerging as key mediators in the establishment of skeletal metastases associated with PCa.
The term “macrophage,” coming from the Greek root “macro,” meaning “large,” and “phage,” “to eat,” characterizes the most well-known activity of macrophages, which is to engulf pathogens. Macrophages serve key roles in host defense in both innate and adaptive immunity. There are multiple subsets of macrophages that demonstrate heterogeneity based on their strategic location in the body tissues and respective microenvironmental cues. Despite extensive and longstanding study of macrophages in a variety of organs, very little is known about macrophage function in the bone marrow microenvironment. Wound healing is a central macrophage task, and since cancer has been termed “a wound that never heals,” a role for macrophages in cancer and in the bone marrow microenvironment of the metastatic lesion is without doubt, yet underinvestigated. Bone-resident macrophages termed “osteomacs” are found in close association with osteoblasts, and are important for bone remodeling, repair, and hematopoietic stem cell niche maintenance.113,114 Winkler et al. utilized two different models of macrophage depletion to demonstrate the impact of macrophages in the hematopoietic stem cell niche.115 Both the Mafia (macrophage Fas-induced apoptosis) model and a clodronate liposome approach that depleted macrophages resulted in HSC mobilization into the blood, and support the role of bone marrow macrophages in endosteal HSC niche maintenance. Similar strategies were used to demonstrate the integral role of “osteomacs” in fracture healing where these macrophages support osteoblastic bone formation.116 Macrophages are constantly surveying their surroundings for signs of tissue damage or invaders, and their precursors rapidly mobilize to sites of trauma or infection. Tumor cells invading the bone marrow are likely targets for “osteomacs,” and their interaction with osteoblasts is an intriguing aspect of the tumor microenvironment in prostate cancer skeletal metastasis.
B. MDSCs and TAMs
In the focused context of cancer, the most well-known roles for cells of the myeloid lineage are for myeloid derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs).117,118 Macrophages can be additionally segregated to M1 proinflammatory macrophages and M2 protumorigenic macrophages.119 It is likely the balance of these two cell types that drives tumor localization in metastatic sites including the bone. This is an area of great interest, and promises to provide a further depth of knowledge regarding the bone marrow niche as well as targetable approaches to addressing skeletal metastasis. Indeed, a role for macrophages in cancer has long been known, and most recently is emerging to be more complex than originally thought with actions both supporting and combating cancer.120,121 Relative to skeletal metastasis, very little is known, yet evidence is strong and building. Clinical studies in prostate cancer demonstrated that overexpression of colony-stimulating factor (CSF-1) and its receptor (CSF-1R or c-fms), responsible for monocyte and macrophage expansion, as well as the CD68 phagocytic cell marker, indicated poor prognosis in primary prostate cancers and development of metastasis to bone.122
TAMs have been implicated in tumor growth, progression, and metastasis of different types of cancer, and emerging evidence supports their role in skeletal metastasis.117,123,124 Nearly 20 years ago, TAMs isolated from lung metastases were reported to have the ability to resorb bone, implicating them in the process of skeletal metastasis. 125 Later, and in the context of prostate cancer, CD11b+ cells were also found capable of differentiating to osteoclastic cells.126 Macrophages have also been shown to stimulate neuroendocrine differentiation of prostate cancer cells via their production of interleukin-6.127 One of the most well-characterized roles of TAMs is in angiogenesis, where they are recruited to angiogenic sites to support the establishment of new vessels by secreting angiogenic factors and proteases. 128 More recently, myeloid lineage cells have been found to support tumor-associated lymphatic vessels and hence a route for metastatic dissemination.129
VII. MYELOID CELLS AND THE PREMETASTATIC NICHE
The concept of a premetastatic niche, originated with studies performed by Lyden’s group, largely focused on the lung.130,131 The underlying tenet of the premetastatic niche is that primary tumors secrete factors that circulate in an endocrine manner to the metastatic site where they condition the “soil” to be receptive for tumor cell localization and growth. Alternatively, a tumor cell that has already circulated to a metastatic site could secrete paracrine factors that condition the niche to provide a convivial environment for tumor expansion. Central to much of the work in this area are myeloid lineage cells. Mobilization of bone marrow–derived myeloid cells (VEGFR1+CD11b+ cells) occurs on production of inflammatory chemokines produced by the primary tumor.131 The myeloid cells circulate to the primary tumor site and collaborate with other cells types in the premetastatic site such as stromal and endothelial cells to produce growth factors, matrix metalloproteinases, and adhesion factors that support metastatic localization and growth.
The fertile ground of the bone marrow for myeloid cells suggests the bone would be a prime example of a premetastatic niche. This has not yet been widely validated, yet is an area of emerging interest. Precise and agreed-upon characterization of the specific myeloid population cell types has limited this area from more rapid progress. Perhaps one of the best examples of the bone as a premetastatic niche for prostate cancer stems from work performed to elucidate the role of parathyroid hormone–related protein (PTHrP) in skeletal metastasis. PTHrP is a tumor-derived protein with widespread autocrine, intracrine, and paracrine actions. It was originally identified as an etiologic factor in cancer-induced hypercalcemia.132 Humoral hypercalcemia of malignancy (HHM) represents the endocrine potential of PTHrP that results when a primary tumor produces PTHrP that circulates to the bone and results in bone resorption. For many years, it was thought that cancers produced ectopic PTH since the skeletal impact was similar to that of hyperparathyroidism. PTH and PTHrP bind to receptors on osteoblasts lining endosteal surfaces, and when levels are continuously high, the osteoblasts support osteoclastogenesis via altering the ratio of RANKL and OPG to support osteoclast differentiation and activation. Interestingly, many cancers that produce PTHrP have very low or undetectable levels of PTHrP in circulation (e.g., prostate cancer), yet evidence is building that there may indeed be a biological impact of this PTHrP relative to a premetastatic niche. Prostate cancer–derived PTHrP has been shown to stimulate osteoblasts to produce chemokine (C-C motif) ligand 2 (CCL2), also known as monocyte chemotactic protein 1 (MCP1).133 CCL2 in turn recruits monocytes, stimulates osteoclastogenesis, and promotes vasculogenesis to support tumor growth.134 Blocking CCL2 effectively reduced tumor growth in skeletal sites. These data suggest that circulating tumor–derived PTHrP may condition the niche via myeloid lineage cells, rendering it a more favorable environment for metastatic tumor cells.
Further evidence stems from work in breast cancer where lysyl oxidase secreted by breast cancer cells accumulates at premetastatic sites and recruits CD11b+ myeloid cells.135 The CD11b+ cells produce matrix metalloproteinases that degrade the extracellular matrix and enhance the invasion of metastasizing tumor cells. Depletion of myeloid cells in an aggressive mammary tumor model via the use of clodronate liposomes effectively reduced metastasis to the lung. A targeting strategy under investigation for prostate cancer directed at the VEGFR effectively reduced osteoblastic tumor progression by targeting angiogenesis and preosteoclastic cells.136 Because myeloid lineage cells bear the VEGFR, this raises the question as to how much of the prostate cancer phenotype is supported by myeloid lineage cells.
A. Macrophages as Phagocytes: A New Role in Cancer
Macrophages are key cellular mediators of infection and inflammation, well known outside of the bone environment for their ability to phagocytose invaders in performing their protective functions.112 The term “efferocytosis” refers to the specific phagocytosis of apoptotic cells and is an integral process in tissue homeostasis, inflammation, autoimmunity, and cancer.137 Macrophages utilize distinct receptor signaling pathways to identify apoptotic cells for phagocytosis.138 Receptor protein kinases including TYRO3, AXL, and MER function to provide identification of “eat me” signals on apoptotic cells via their ligands such as growth arrest–specific 6 (GAS6) or protein S.139 Ablation of MER via c-mer proto-oncogene tyrosine kinase (Mertk) receptor mutation reduces efferocytosis and promotes apoptotic cell accumulation, and presents a promising yet untested approach to disrupt the macrophage efferocytosis cycle.140 When macrophages encounter apoptotic cells, Gas6 facilitates the interaction of the apoptotic cell membrane lipid phosphatidylserine (PS) and the macrophage MER to increase phagocytosis and clearance of apoptotic cells. The intracellular signaling for Mertk has been proposed with phosphorylation of the Tyr-867, leading to PLCg2 activation, activation of PKC, and PKC-mediated tyrosine phosphorylation of FAK.141 FAK phosphorylation leads to cytoskeletal rearrangements essential for macrophage phagocytosis. Such intracellular signaling events have been shown to be specifically related to the phagocytosis of apoptotic cells and represent intriguing new pharmacologic targets. Importantly, when macrophages phagocytose apoptotic cells, they release TGFβ, a growth factor well known for supporting tumor growth.142–144 TGFβ also recruits mesenchymal stem cells to support osteoblastic activity in bone,145 and may be implicated in the osteoblastic lesions associated with prostate cancer.
Findings that macrophages play detrimental roles in tumorigenesis lead to consideration of targetable approaches to reduce their pathogenicity. Indeed, bisphosphonates that are widely used in skeletal metastasis show evidence of their efficacy via targeting macrophages.146 As more and more interest builds in this area, new therapeutics will likely arise that focus on specific aspects of myeloid cell function in the support of tumor cell localization and growth in the bone marrow microenvironment.
VIII. SUMMARY
It is clear that multiple mechanisms contribute to PCa cells’ ability to parasitize the bone (Fig. 1). Although research in the field has contributed much toward developing a basic understanding of the events that lead toward establishment of metastases, there are still many gaps in knowledge. Large gains in understanding of the biology of HSCs and their niche have provided a key framework toward developing mechanisms of PCa bone metastasis. The classical case of patients developing bone metastases long after a primary tumor is resected clearly demonstrates the important of DTC. Furthermore, DTCs must have properties of CSCs, although perhaps the term TIC is more appropriate. The ability of DTCs to compete for the HSC provides a potential explanation for how these DTCs exist in a dormant state. Eventually, changes may occur in either the DTCs themselves and/or the bone microenvironment that allows escape from dormancy and tumor growth. These changes can include both locally derived cytokines and cells, such as myeloid cells, or distant cells, such as TAMs, that modulate the bone microenvironment. As we continue to define the mechanisms of bone metastasis, we will identify potential therapeutic targets that will help men afflicted with PCa.
FIGURE 1.
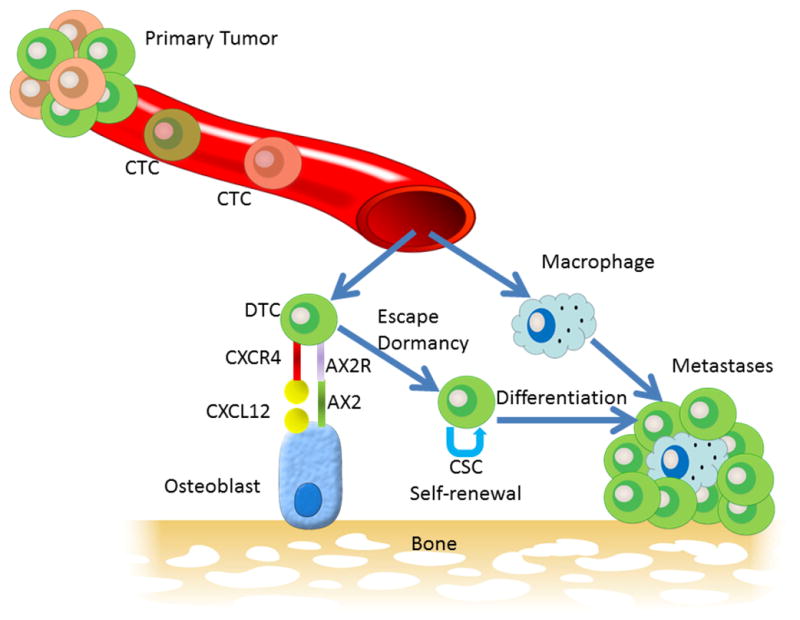
Model of the pathway to parasitism of the bone microenvironment by prostate cancer. Primary tumors represent a heterogeneous population of cancer cells, some of which intravasate into the blood circulation to become circulating tumor cells (CTCs). A subset of CTCs may successfully target the bone microenvironment through such mechanisms as chemotaxis toward CXCL12 (also called stromal-derived factor) through its receptor CXCR4 and then docking to osteoblasts annexin II receptor (AX2R) binding to annexin II (AX2). On docking to the osteoblasts, the PCa cells, similar to hematopoietic stem cells, enter a dormant state. Multiple changes, perhaps in the PCa cells themselves and/or in the bone microenvironment, result in escape from dormancy. The PCa cells may then act as a CSC undergoing both self-renewal and differentiation into clinically detectable metastasis. Host cells, such as macrophages, enter the tumor microenvironment and, through both cell-to-cell interactions and release of various cytokines, influence the progression of tumor growth at the metastatic site.
Acknowledgments
This work was supported by National Institutes of Health Grant No. P01 CA093900.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 2.Pienta KJ, McGregor N, Axelrod R, Axelrod DE. Ecological therapy for cancer: defining tumors using an ecosystem paradigm suggests new opportunities for novel cancer treatments. Transl Oncol. 2008 Dec;1(4):158–64. doi: 10.1593/tlo.08178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odum EPBG, editor. Fundamental of ecology. 5. Belmont, CA: Thompson Brooks/Cole; 2005. [Google Scholar]
- 4.Pienta KJ, Loberg R. The “emigration, migration, and immigration” of prostate cancer. Clin Prostate Cancer. 2005 Jun;4(1):24–30. doi: 10.3816/cgc.2005.n.008. [DOI] [PubMed] [Google Scholar]
- 5.Sakai AK, Holt JS, Lodge DM, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, Mc-Cauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annu Rev Ecol Syst. 2001;32:305–32. [Google Scholar]
- 6.Lodge DM. Biological invasions: Lessons for ecology. Trends Ecol Evol. 1993 Apr;8:133–7. doi: 10.1016/0169-5347(93)90025-K. [DOI] [PubMed] [Google Scholar]
- 7.Williamson MBK. The analysis and modeling of British invasions. Phil Trans R Soc Lond. 1986;314(B):505–22. [Google Scholar]
- 8.Baker H. The evolution of weeds. Annu Rev Ecol Syst. 1974;5:1–24. [Google Scholar]
- 9.Kolar CS, Lodge DM. Progress in invasion biology: predicting invaders. Trends Ecol Evol. 2001 Apr 1;16(4):199–204. doi: 10.1016/s0169-5347(01)02101-2. [DOI] [PubMed] [Google Scholar]
- 10.Newsome AENI, editor. Ecological and physiological characters of invading species. Cambridge, England: Cambridge University Press; 1986. [Google Scholar]
- 11.MJC The population biology of invaders. Phil Trans R Soc Lond. 1986;314(B):711–29. [Google Scholar]
- 12.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011 Mar 25;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Murray NP, Reyes E, Tapia P, Orellana N, Duenas R, Fuentealba C, Badinez L. Diagnostic performance of malignant prostatic cells detection in blood for early detection of prostate cancer: comparison to prostatic biopsy. Arch Esp Urol. 2011 Dec;64(10):961–71. [PubMed] [Google Scholar]
- 15.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003 Feb;61(2):277–81. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 16.Pfitzenmaier J, Ellis WJ, Hawley S, Arfman EW, Klein JR, Lange PH, Vessella RL. The detection and isolation of viable prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol Oncol. 2007 May-Jun;25(3):214–20. doi: 10.1016/j.urolonc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Gao CL, Dean RC, Pinto A, Mooneyhan R, Connelly RR, McLeod DG, Srivastava S, Moul JW. Detection of circulating prostate specific antigen expressing prostatic cells in the bone marrow of radical prostatectomy patients by sensitive reverse transcriptase polymerase chain reaction. J Urol. 1999 Apr;161(4):1070–6. [PubMed] [Google Scholar]
- 18.Riethdorf S, Pantel K. Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann N Y Acad Sci. 2010 Oct;1210:66–77. doi: 10.1111/j.1749-6632.2010.05779.x. [DOI] [PubMed] [Google Scholar]
- 19.Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010 Sep;16(9):398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Lin H, Balic M, Zheng S, Datar R, Cote RJ. Disseminated and circulating tumor cells: Role in effective cancer management. Crit Rev Oncol Hematol. 2011 Jan;77(1):1–11. doi: 10.1016/j.critrevonc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Klein CA, Blankenstein TJ, Schmidt-Kittler O, Petronio M, Polzer B, Stoecklein NH, Riethmuller G. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002 Aug 31;360(9334):683–9. doi: 10.1016/S0140-6736(02)09838-0. [DOI] [PubMed] [Google Scholar]
- 22.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001 Nov 1;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 23.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer. 2005 Apr;5(4):311–21. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 24.Clarke M. Cancer stem cells—perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–44. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 25.Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009 Aug;15(8):907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 26.Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, Jeter C. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Mol Carcinog. 2007 Jan;46(1):1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 27.Liu AY, True LD, LaTray L, Nelson PS, Ellis WJ, Vessella RL, Lange PH, Hood L, van den Engh G. Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc Natl Acad Sci U S A. 1997;94(20):10705–10. doi: 10.1073/pnas.94.20.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002 Jun 24;157(7):1257–65. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawson DA, Witte ON. Stem cells in prostate cancer initiation and progression. J Clin Invest. 2007 Aug;117(8):2044–50. doi: 10.1172/JCI32810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11(3):229–42. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- 31.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005 Dec 1;65(23):10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 32.Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A. 2007 Jan 2;104(1):181–6. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao CP, Adisetiyo H, Liang M, Roy-Burman P. Cancer-associated fibroblasts enhance the gland-forming capability of prostate cancer stem cells. Cancer Res. 2010 Sep 15;70(18):7294–303. doi: 10.1158/0008-5472.CAN-09-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1480–5. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maitland NJ, Frame FM, Polson ES, Lewis JL, Collins AT. Prostate cancer stem cells: do they have a basal or luminal phenotype? Horm Cancer. 2011 Feb;2(1):47–61. doi: 10.1007/s12672-010-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010 Jul 30;329(5991):568–71. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci U S A. 2010 Feb 9;107(6):2610–5. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005 Oct 28;310(5748):644–8. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 39.Birnie R, Bryce SD, Roome C, Dussupt V, Droop A, Lang SH, Berry PA, Hyde CF, Lewis JL, Stower MJ, Maitland NJ, Collins AT. Gene expression profiling of human prostate cancer stem cells reveals a pro-inflammatory phenotype and the importance of extracellular matrix interactions. Genome Biol. 2008;9(5):R83. doi: 10.1186/gb-2008-9-5-r83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajasekhar VK, Studer L, Gerald W, Socci ND, Scher HI. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat Commun. 2011 Jan 18;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korsten H, Ziel-van der Made A, Ma X, van der Kwast T, Trapman J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS One. 2009;4(5):e5662. doi: 10.1371/journal.pone.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009 Sep 24;461(7263):495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006 Mar 16;25(12):1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 44.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008 Feb 26;98(4):756–65. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang LS, Yang Q, Huang J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009 May 15;69(7):787–98. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 46.Rybak AP, He L, Kapoor A, Cutz JC, Tang D. Characterization of sphere-propagating cells with stem-like properties from DU145 prostate cancer cells. Biochim Biophys Acta. 2011 May;1813(5):683–94. doi: 10.1016/j.bbamcr.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. J Cell Sci. 2004 Jul 15;117(Pt 16):3539–45. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- 48.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcinoma cells DU145: the seeds of the cell line? Cancer Biol Ther. 2007 May;6(5):763–8. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 49.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008 Dec 1;68(23):9703–11. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiffer MJ, Schalken JA. Stem cell characteristics in prostate cancer cell lines. Eur Urol. 2010 Feb;57(2):246–54. doi: 10.1016/j.eururo.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida A, Hsu LC, Dave V. Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase. Enzyme. 1992;46(4–5):239–44. doi: 10.1159/000468794. [DOI] [PubMed] [Google Scholar]
- 52.Li T, Su Y, Mei Y, Leng Q, Leng B, Liu Z, Stass SA, Jiang F. ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Lab Invest. 2010 Feb;90(2):234–44. doi: 10.1038/labinvest.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burger PE, Gupta R, Xiong X, Ontiveros CS, Salm SN, Moscatelli D, Wilson EL. High aldehyde dehydrogenase activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells. 2009 Sep;27(9):2220–8. doi: 10.1002/stem.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Hoogen C, van der Horst G, Cheung H, Buijs JT, Lippitt JM, Guzman-Ramirez N, Hamdy FC, Eaton CL, Thalmann GN, Cecchini MG, Pelger RC, van der Pluijm G. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010 Jun 15;70(12):5163–73. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 55.Yu C, Yao Z, Dai J, Zhang H, Escara-Wilke J, Zhang X, Keller ET. ALDH activity indicates increased tumorigenic cells, but not cancer stem cells, in prostate cancer cell lines. In Vivo. 2011 Jan-Feb;25(1):69–76. [PubMed] [Google Scholar]
- 56.Pellegrini G, Ranno R, Stracuzzi G, Bondanza S, Guerra L, Zambruno G, Micali G, De Luca M. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous kerati-nocytes cultured on fibrin. Transplantation. 1999 Sep 27;68(6):868–79. doi: 10.1097/00007890-199909270-00021. [DOI] [PubMed] [Google Scholar]
- 57.Doherty RE, Haywood-Small SL, Sisley K, Cross NA. Aldehyde dehydrogenase activity selects for the holoclone phenotype in prostate cancer cells. Biochem Biophys Res Commun. 2011 Nov 4;414(4):801–7. doi: 10.1016/j.bbrc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 58.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994 May 1;179(5):1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taichman RS. Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood. 2005 Apr 1;105(7):2631–9. doi: 10.1182/blood-2004-06-2480. [DOI] [PubMed] [Google Scholar]
- 60.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004 Jul 23;118(2):149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 61.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003 Oct 23;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003 Oct 23;425(6960):836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 63.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005 Jul 1;121(7):1109–21. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 64.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009 Jul 9;460(7252):259–63. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010 Aug 12;466(7308):829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006 Dec;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 68.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010 Apr 8;464(7290):852–7. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li P, Zon LI. Resolving the controversy about N-cadherin and hematopoietic stem cells. Cell stem cell. 2010 Mar 5;6(3):199–202. doi: 10.1016/j.stem.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 70.Askmyr M, Sims NA, Martin TJ, Purton LE. What is the true nature of the osteoblastic hematopoietic stem cell niche? Trends in endocrinology and metabolism: TEM. 2009 Aug;20(6):303–9. doi: 10.1016/j.tem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006 May;116(5):1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007 May 1;109(9):3706–12. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 73.Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004 May 1;103(9):3258–64. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 74.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell stem cell. 2007 Dec 13;1(6):685–97. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, Thoren LA, Ekblom M, Alexander WS, Jacobsen SE. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007 Dec 13;1(6):671–84. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 76.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp M. 2005 Jun 6;201(11):1781–91. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005 Aug 15;106(4):1232–9. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 78.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999 Feb 5;283(5403):845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 79.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T. GCSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002 Jul;3(7):687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 80.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005 Apr 18;201(8):1307–18. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006 Jan 27;124(2):407–21. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 82.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008 Mar 27;452(7186):442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 83.Jung Y, Wang J, Song J, Shiozawa Y, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007 Jul 1;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006 Feb 2;439(7076):599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 85.Adams GB, Alley IR, Chung UI, Chabner KT, Jeanson NT, Lo Celso C, Marsters ES, Chen M, Weinstein LS, Lin CP, Kronenberg HM, Scadden DT. Haematopoietic stem cells depend on Galpha(s)-mediated signalling to engraft bone marrow. Nature. 2009 May 7;459(7243):103–7. doi: 10.1038/nature07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007 Nov 23;318(5854):1296–9. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Priestley GV, Scott LM, Ulyanova T, Papayannopoulou T. Lack of alpha4 integrin expression in stem cells restricts competitive function and self-renewal activity. Blood. 2006 Apr 1;107(7):2959–67. doi: 10.1182/blood-2005-07-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001 Oct 15;98(8):2403–11. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 89.Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, de Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nature medicine. 2004 Jan;10(1):64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- 90.Jung Y, Song J, Shiozawa Y, Wang J, Wang Z, Williams B, Havens A, Schneider A, Ge C, Franceschi RT, McCauley LK, Krebsbach PH, Taichman RS. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008 Aug;26(8):2042–51. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin Couples Hematopoiesis with Bone Formation. PLoS One. 2010;5(5):e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morgan TM, Lange PH, Vessella RL. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- 93.Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells, and unwitting host to molecular parasites. Leukemia. 2008 May;22(5):941–50. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009 Apr;9(4):239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002 Mar 15;62(6):1832–7. [PubMed] [Google Scholar]
- 96.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001 Mar 1;410(6824):50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 97.Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008 Oct 1;105(2):370–80. doi: 10.1002/jcb.21835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. The American journal of pathology. 1996 Jan;148(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 99.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011 Apr 1;121(4):1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, Neeley C, Wang J, Mehra R, Keller ET, Mc-Cauley LK, Loberg RD, Pienta KJ, Taichman RS. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008 Apr;10(4):371–80. doi: 10.1593/neo.08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pantel K, Alix-Panabieres C, Riethdorf S. Cancer micro-metastases. Nat Rev Clin Oncol. 2009 Jun;6(6):339–51. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 102.Morgan TM, Lange PH, Porter MP, Lin DW, Ellis WJ, Gallaher IS, Vessella RL. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009 Jan 15;15(2):677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nash KT, Phadke PA, Navenot JM, Hurst DR, Accavitti-Loper MA, Sztul E, Vaidya KS, Frost AR, Kappes JC, Peiper SC, Welch DR. Requirement of KISS1 secretion for multiple organ metastasis suppression and maintenance of tumor dormancy. J Natl Cancer Inst. 2007 Feb 21;99(4):309–21. doi: 10.1093/jnci/djk053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Townson JL, Chambers AF. Dormancy of solitary metastatic cells. Cell Cycle. 2006 Aug;5(16):1744–50. doi: 10.4161/cc.5.16.2864. [DOI] [PubMed] [Google Scholar]
- 105.Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolayers that overexpress growth arrest-specific gene-6 (GAS6) Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12260–5. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Neubauer A, Fiebeler A, Graham DK, O’Bryan JP, Schmidt CA, Barckow P, Serke S, Siegert W, Snodgrass HR, Huhn D. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994 Sep 15;84(6):1931–41. [PubMed] [Google Scholar]
- 107.Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Liu ET. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010 Feb;12(2):116–27. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shiozawa Y, Pedersen EA, Taichman RS. GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp Hematol. 2010 Feb;38(2):132–40. doi: 10.1016/j.exphem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bhatia M, Bonnet D, Wu D, Murdoch B, Wrana J, Gallacher L, Dick JE. Bone morphogenetic proteins regulate the developmental program of human hematopoietic stem cells. J Exp M. 1999 Apr 5;189(7):1139–48. doi: 10.1084/jem.189.7.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011 Dec 19;208(13):2641–55. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–3. [PubMed] [Google Scholar]
- 112.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011 Nov;11(11):788–98. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 113.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008 Jul 15;181(2):1232–44. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 114.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011 Feb 14;208(2):261–71. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Levesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010 Dec 2;116(23):4815–28. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 116.Alexander KA, Chang MK, Maylin ER, Kohler T, Muller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Mineral Res. 2011 Jul;26(7):1517–32. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 117.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004 Jan;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 118.Sica A. Role of tumour-associated macrophages in cancer-related inflammation. Exp Oncol. 2010 Sep;32(3):153–8. [PubMed] [Google Scholar]
- 119.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008 Oct;18(5):349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 120.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010 Apr;22(2):231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 121.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011 Nov;11(11):723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Richardsen E, Uglehus RD, Due J, Busch C, Busund LT. The prognostic impact of M-CSF, CSF-1 receptor, CD68 and CD3 in prostatic carcinoma. Histopathology. 2008 Jul;53(1):30–8. doi: 10.1111/j.1365-2559.2008.03058.x. [DOI] [PubMed] [Google Scholar]
- 123.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011 Jun;11(6):411–25. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009 Apr;9(4):259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Athanasou NA, Quinn JM. Human tumour-associated macrophages are capable of bone resorption. Br J Cancer. 1992 Apr;65(4):523–6. doi: 10.1038/bjc.1992.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mizutani K, Sud S, Pienta KJ. Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts. J Cell Biochem. 2009 Mar 1;106(4):563–9. doi: 10.1002/jcb.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee GT, Kwon SJ, Lee JH, Jeon SS, Jang KT, Choi HY, Lee HM, Kim WJ, Lee DH, Kim IY. Macrophages induce neuroendocrine differentiation of prostate cancer cells via BMP6-IL6 Loop. Prostate. 2011 Mar 3; doi: 10.1002/pros.21369. [DOI] [PubMed] [Google Scholar]
- 128.Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX, Kong LQ, Wang L, Wu WZ, Tang ZY. Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimeta-static and antiangiogenic effects. Clin Cancer Res. 2010 Jul 1;16(13):3420–30. doi: 10.1158/1078-0432.CCR-09-2904. [DOI] [PubMed] [Google Scholar]
- 129.Zumsteg A, Baeriswyl V, Imaizumi N, Schwendener R, Ruegg C, Christofori G. Myeloid cells contribute to tumor lymphangiogenesis. PloS One. 2009;4(9):e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metast Rev. 2006 Dec;25(4):521–9. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 131.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005 Dec 8;438(7069):820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, Diefenbach-Jagger H, Rodda CP, Kemp BE, Rodriguez H, Chen EY, Hudson PJ, Martin TJ, Wood WI. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987 Aug 21;237(4817):893–96. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 133.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. The Journal of biological chemistry. 2009 Dec 4;284(49):34342–54. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009 Nov;11(11):1235–42. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009 Jan 6;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohamedali KA, Li ZG, Starbuck MW, Wan X, Yang J, Kim S, Zhang W, Rosenblum MG, Navone NM. Inhibition of prostate cancer osteoblastic progression with VEGF121/rGel, a single agent targeting osteoblasts, osteoclasts, and tumor neovasculature. Clin Cancer Res. 2011 Apr 15;17(8):2328–38. doi: 10.1158/1078-0432.CCR-10-2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mevorach D, Trahtemberg U, Krispin A, Attalah M, Zazoun J, Tabib A, Grau A, Verbovetski-Reiner I. What do we mean when we write “senescence,” “apoptosis,” “necrosis,” or “clearance of dying cells”? Ann NY Acad Sci. 2010 Oct;1209:1–9. doi: 10.1111/j.1749-6632.2010.05774.x. [DOI] [PubMed] [Google Scholar]
- 138.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010 Dec;22(6):740–6. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, Masiakowski P, Ryan TE, Tobkes NJ, Chen DH, DiStefano PS, Long GL, Basilico C, Goldfarb MP, Lemke G, Glass DJ, Yancopoulos GD. The anticoagulation factor protein S and its relative, Gas6, are ligands for the Tyro 3/Axl family of receptor tyrosine kinases. Cell. 1995 Feb 24;80(4):661–70. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 140.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008 Aug;28(8):1421–8. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tibrewal N, Wu Y, D’Mello V, Akakura R, George TC, Varnum B, Birge RB. Autophosphorylation docking site Tyr-867 in Mer receptor tyrosine kinase allows for dissociation of multiple signaling pathways for phagocytosis of apoptotic cells and down-modulation of lipopolysaccharide-inducible NF-kappaB transcriptional activation. J Biol Chem. 2008 Feb 8;283(6):3618–27. doi: 10.1074/jbc.M706906200. [DOI] [PubMed] [Google Scholar]
- 142.Nacu N, Luzina IG, Highsmith K, Lockatell V, Pochetuhen K, Cooper ZA, Gillmeister MP, Todd NW, Atamas SP. Macrophages produce TGF-beta-induced (beta-igh3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol. 2008 Apr 1;180(7):5036–44. doi: 10.4049/jimmunol.180.7.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Juarez P, Guise TA. TGF-beta in cancer and bone: implications for treatment of bone metastases. Bone. 2011 Jan;48(1):23–9. doi: 10.1016/j.bone.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 144.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010 Feb;21(1):49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z, Zhao L, Nagy TR, Peng X, Hu J, Feng X, Van Hul W, Wan M, Cao X. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med. 2009 Jul;15(7):757–65. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transla Med. 2011;9:177. doi: 10.1186/1479-5876-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]


