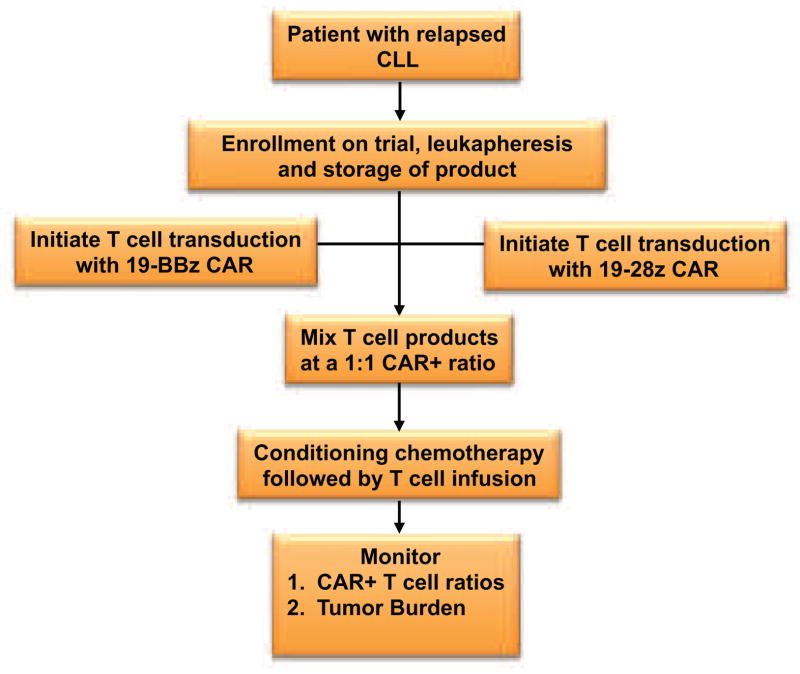
FIGURE 4. MSKCC and UPenn Treatment Schema comparing 19-28z and 19-BBz CAR-modified T cells in patients with relapsed CLL.
Patients with relapsed CLL at MSKCC or UPenn are eligible for enrollment, leukapheresis, and infusion with CAR-modified T cells after treatment with conditioning chemotherapy. The patients are infused with a mixture of CAR-modified T cells composed of an equal ratio of 19-28z+ T cells to 19-BBz+ T cells. Transduction of the 19-28z CAR occurs by gammaretroviral transduction, while transduction of the 19-BBz CAR occurs by lentiviral transduction. Production of both T cell groups occurs at the GMP facility located within the medical center treating the patient. Enhanced T cell persistence and/or proliferation will be determined by measuring the ratio of both CAR-modified T cell groups in treated patients.