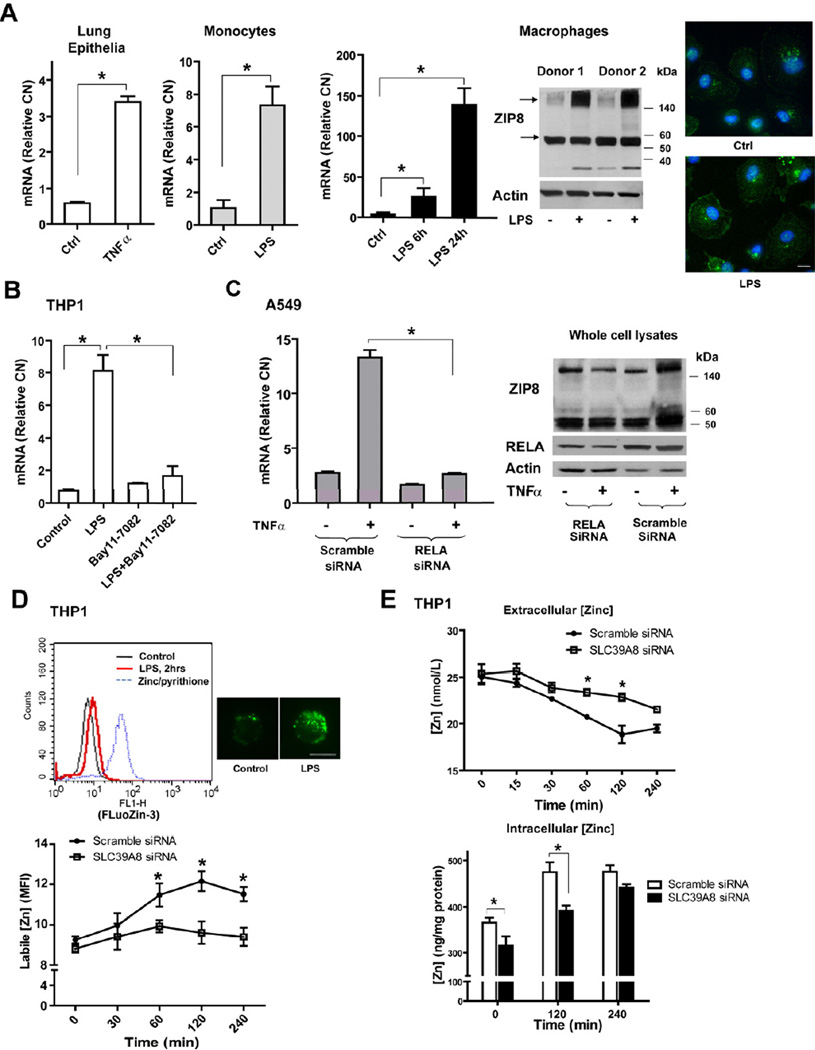
Figure 1. SLC39A8 expression is induced by pro-inflammatory stimuli in a NF-κB-dependent manner and regulates zinc uptake.
(A) Human primary lung epithelia were treated with TNFα (10 ng/mL) for 16 hrs (n=3); human primary monocytes were treated with LPS 1.µg/mL) for 4 hrs (n=4); CD14+ monocyte-derived primary macrophages were treated with LPS (1 µg/mL) for 6 and 24 hrs. SLC39A8 mRNA levels were determined by real-time PCR analysis (Relative CN: relative copy number). Western blot was performed on membrane fractions with ZIP8 antiserum. Immunofluorescence staining shows ZIP8 localization (green) in macrophages, counterstained with DAPI (Scale bar: 10 µm). (B) THP1 cells were treated with LPS (1 µg/mL) for 4 hrs with Bay11–7082 (20 µM), added 30 min prior to LPS. (C) A549 cells were treated with TNFα (10 ng/mL) in combination with RELA siRNA (40 pmol), which was transfected 24 hrs before TNFα treatment. (D) Control siRNA or SLC39A8 siRNA -treated THP1 cells were stimulated with LPS (1 µg/mL), followed by FluoZin-3 staining. Intracellular labile zinc was quantified by flow cytometry (MFI, Mean fluorescence intensity). Representative composite flow cytometric histograms are shown and ZnSO4 (10 µM) /pyrithione (10 µM) served as a positive control. Two representative FluoZin-3 staining images are shown (Scale bar: 10 µm). Western analysis of ZIP8 silencing is shown in Figure 3A. (E) Total zinc levels in culture medium and cell pellets were detected by AAS or ICP-OES, respectively.