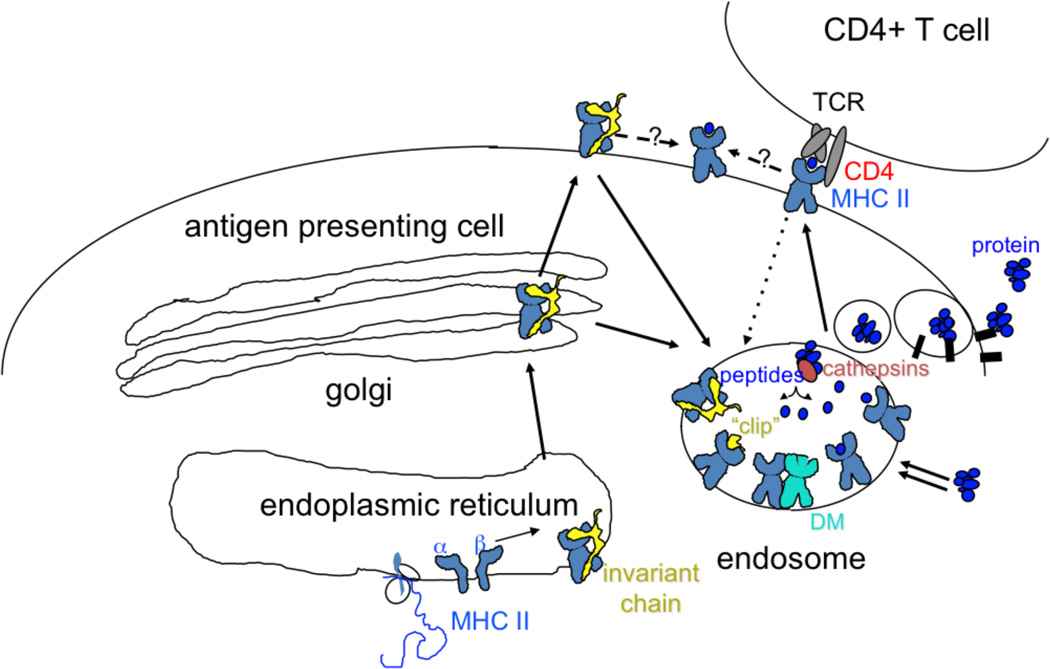
Figure 1.
Class II MHC antigen processing pathway. MHC class II alpha and beta subunits associate with the invariant chain chaperone, which escorts them to an endosomal compartment, perhaps via transient appearance at the cell surface. Proteins destined for processing also arrive at the endosome, by fluid-phase or receptor mediated uptake, or by autophagy (double arrows). Endosomal proteases (cathepsins) cleave the invariant chain, leaving a small remnant “clip” in the MHC peptide binding site. Proteins are also degraded by endosomal cathepsins, perhaps aided by oxidoreductases and unfoldases. HLA-DM catalyzes exchange of clip for endosomal peptides (or partially degraded proteins). Fully processed MHC-peptide complexes traffic to the surface for interaction with TCR on CD4+ T cells. Surface MHC-peptide complexes can recycle to endosomes for reloading (dotted line), or can exchange peptides at the surface (dashed lines).