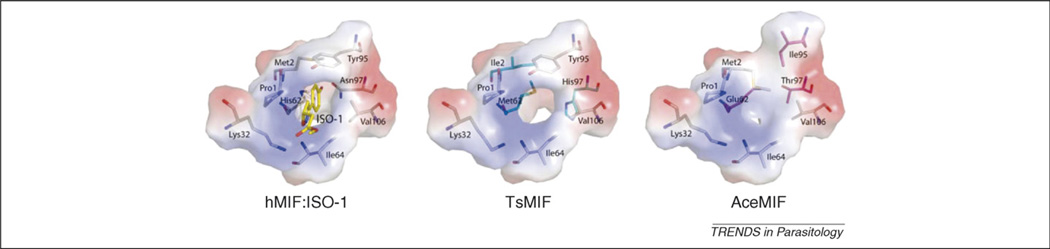
Figure 4.
Distinct amino acid composition in the active sites of human and nematode MIFs. The active site residues located within non-covalent interaction distances to the hMIF inhibitor ISO-1 are represented in stick model in each MIF with the corresponding electrostatic surface potential. The crystallographically determined structure of ISO-1 is colored in yellow with hMIF (human MIF) on the left. The active site of TsMIF (Trichinella spiralis) is in the middle with the residues distinct from those of hMIF in cyan. The active site of AceMIF is on the right with the residues distinct from those of hMIF in maroon. The electrostatic surface potential of all the MIFs are scaled equally to each other.