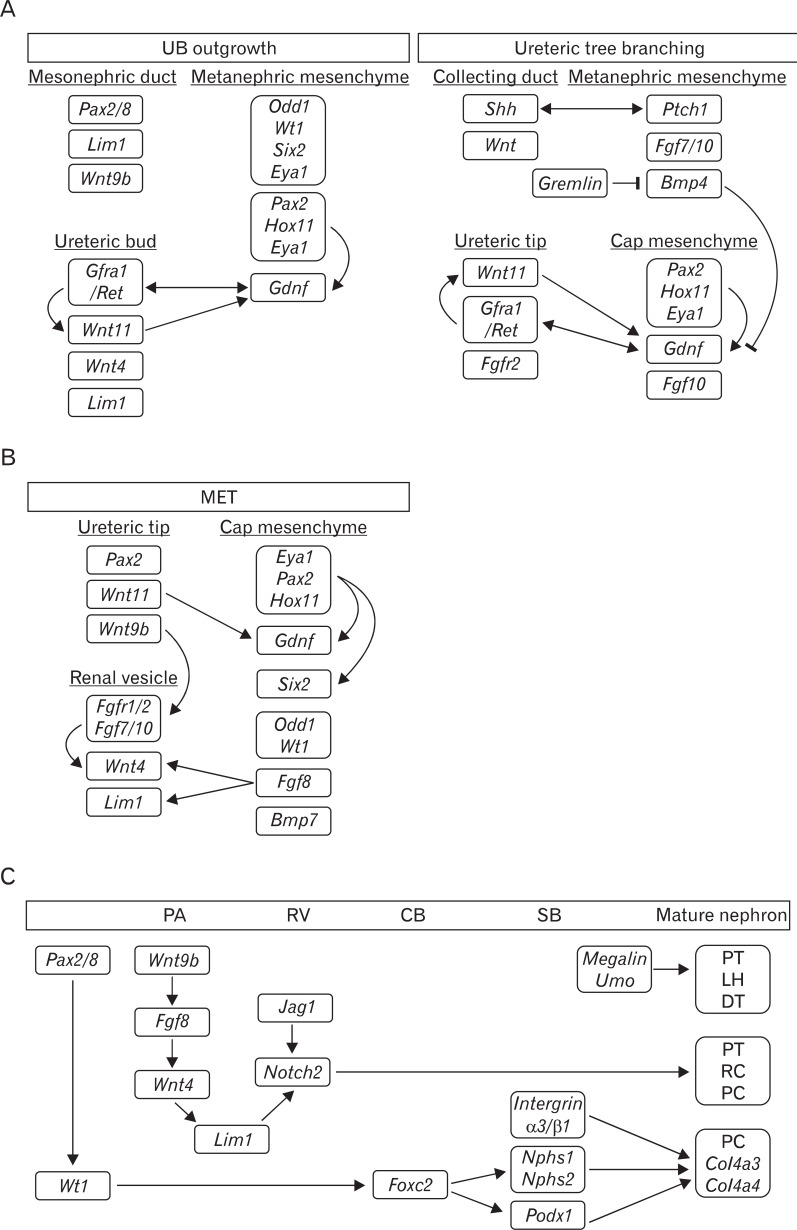
Fig. 4.
Molecular signaling pathways involved in ureteric bud outgrowth and branching (A), mesenchymal-to-epithelial transition (MET) (B), and nephron patterning (C). (A) Molecules and growth factors (Gdnf and Fgfs) from the adjacent cells of the metanephric mesenchyme bind to a variety of receptor-tyrosine kinases (Ret and Fgfr) on the surface of the ureteric tip cell, triggering signaling cascades that regulate cell proliferation, migration, and extracellular matrix degradation. The combined actions of these signaling pathways are continued branching and elongation of the ureteric epithelium to form the collecting duct system. (B) Just as Gdnf/Ret signaling has been central to branching, Wnt signaling is key to kidney development, with both Wnt4 and Wnt9b being involved in MET. In addition to Wnt, Fgfs, Wt1, and Odd1, there are other factors required for MET. Fgf8 is ex pressed earlier than Wnt4 and is ne cessary for both Wnt4 and Lim1. A tripartite complex between Eya1, Pax2, and Hox11 positively regulates the expression of Gdnf and Six2. (C) The transcriptional hierarchy of genes governing nephron pattering is shown. Wnt9b initially activates the expression of Fgf8, Wnt4, and Pax8 in the pre-tubular aggregate (PA), and then Wnt4 maintains the expression of these genes and induces Lim1. Wt1 is restricted to the proximal S-shaped body (SB) and maintained in the podocytes throughout all stages of nephrogenesis. Notch2 is required in the patterning of the pro ximal nephron. In addition, Megalin, Umo, integrin α3/β1, and Nphs play critical roles in the patterning of the loop of Henle (LH), distal tubule (DT), and the formation of functional filters. CB, comma-shaped body; PC, podocyte cells; PT, proximal tubule; RV, renal vesicle; UB, ureteric bud.