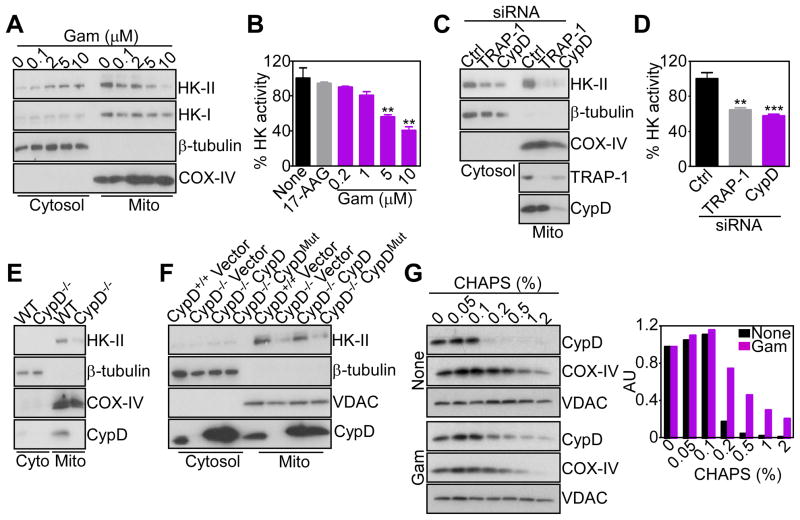
Figure 2. Mitochondrial HSP90s control of CypD folding and HK-II recruitment.
(A) LN229 cells were treated with Gamitrinib (Gam) and cytosolic or mitochondrial (Mito) fractions were analyzed after 5 hr by Western blotting. COX-IV was a mitochondrial marker.
(B) LN229 cells were treated with 17-AAG (10 μM) or Gamitrinib, and mitochondrial fractions were analyzed for hexokinase activity after 5 hr. Mean±SD (n=2); **, p=0.005–0.004.
(C) LN229 cells were transfected with control (Ctrl) or CypD- or TRAP-1-directed siRNA, and isolated mitochondrial (Mito) or cytosol fractions were analyzed after 48 hr by Western blotting.
(D) Mitochondrial fractions from LN229 cells transfected as in (C) were analyzed for hexokinase activity after 48 hr. Mean±SD (n=2); ***, p=0.0009; **, p=0.0024.
(E) Mitochondrial (Mito) or cytosol (Cyto) fractions from WT (CypD+/+) or CypD−/− MEFs were analyzed by Western blotting.
(F) CypD+/+, CypD−/− or CypD−/− MEFs reconstituted with WT or PPIase-defective H168Q mutant CypD cDNA were fractionated in cytosol or mitochondrial (Mito) extracts, and analyzed by Western blotting.
(G) LN229 cells were left untreated (None) or incubated with Gamitrinib (5 μM), mixed with the indicated increasing concentrations of CHAPS, and detergent-insoluble proteins were analyzed by Western blotting. Bar graph: densitometric quantification of protein bands. RU, relative units.