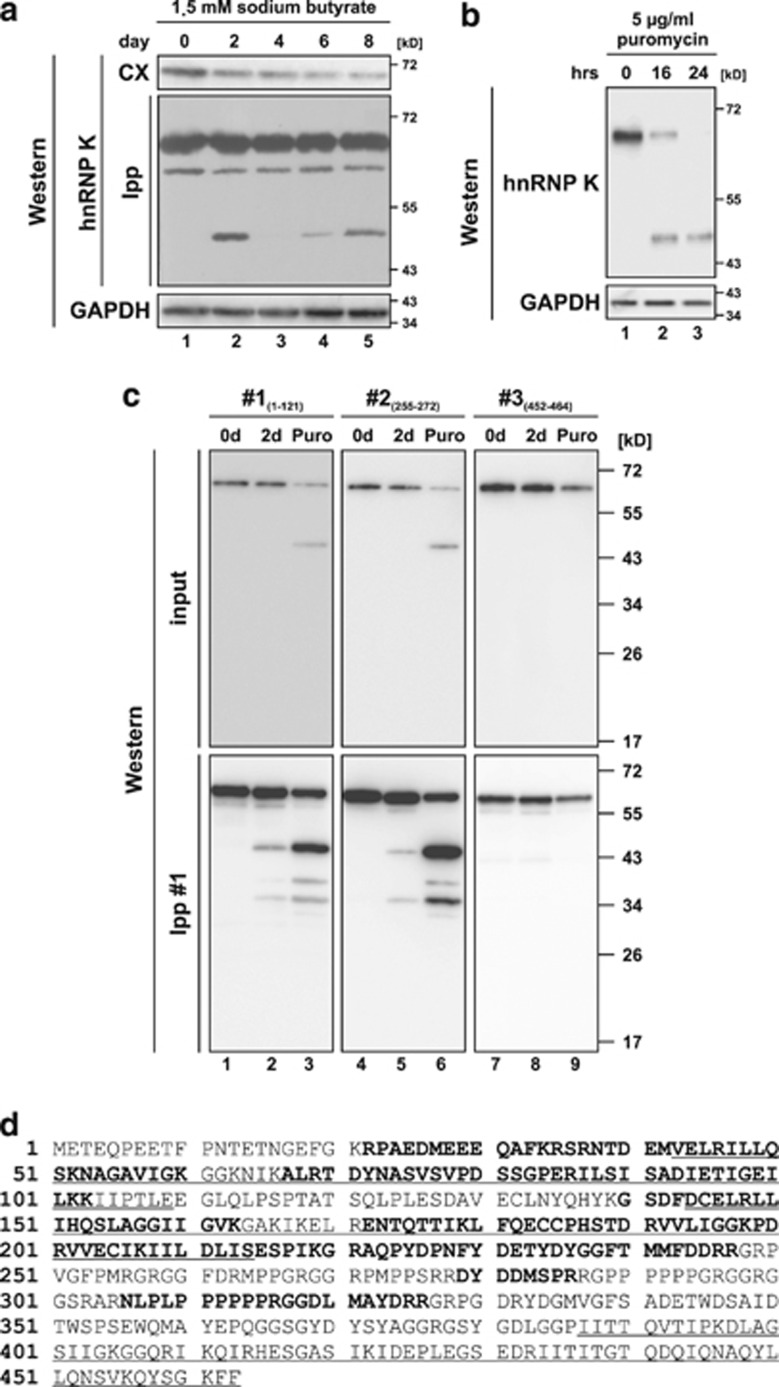
Figure 1.
hnRNP K is degraded via an N-terminal fragment of 48 kD during induced erythroid differentiation and puromycin-induced apoptosis of K562 cells. (a) Detection of hnRNP K in cytoplasmic extract (CX) and immunoprecipitates (immunoprecipitation with hnRNP K antibody #1, Ipp) from K562 cells induced for erythroid differentiation with sodium butyrate for up to 8 days in western blot assays. GAPDH was detected as loading control. (b) Detection of hnRNP K and GAPDH in lysates of K562 cells, which were non-treated or treated with puromycin for 16 or 24 h, respectively. (c) Immunoprecipitation of hnRNP K with antibody #1 from cytoplasmic extracts of non-treated K562 cells (lanes 1, 4 and 7), cells induced with sodium butyrate for 2 days (Lanes 2, 5 and 8) or cells treated with puromycin for 16 h (lanes 3, 6 and 9). Western blot analysis of the input and immunoprecipitates with antibodies #1 (directed against aa 1–121), #2 (directed against aa 255–272) and #3 (directed against aa 452–464). (d) Amino acid sequence of human hnRNP K. The three KH domains are underlined. Peptides identified in the mass spectrometry analysis after trypsin digestion of the purified N-terminal hnRNP K fragment are shown in bold