Abstract
Background
Altered formulations of taxanes may lack cross-resistance with standardly used solvent-based taxanes. The primary objective of the present study was to assess the clinical benefit of nanoparticle albumin-bound (nab)–paclitaxel in women with metastatic breast cancer previously treated with and without adjuvant taxane in British Columbia.
Methods
The BC Cancer Agency Pharmacy data repository and Breast Cancer Outcomes Unit database were linked to identify all patients who received nab-paclitaxel in British Columbia since its introduction in 2007. Hormone receptor status, demographic characteristics, number of cycles prescribed, and time to treatment failure were extracted and analyzed.
Results
From 2007 to 2011, 138 patients in British Columbia received nab-paclitaxel, with 122 patients available for analysis. Most (70.5%) received adjuvant chemotherapy; about a quarter (24.6%) received an adjuvant taxane. Patients who received adjuvant taxane were more likely to have node-positive (86.7% vs. 48.9%, p = 0.007), estrogen receptor–negative (46.7% vs. 13.0% p < 0.001) disease and to receive initial adjuvant radiotherapy (76.7% vs. 51.1%, p < 0.001). For the entire cohort, the median number of nab-paclitaxel cycles prescribed was 4.4 (range: 0.3–13). The median number of nab-paclitaxel cycles was greater when that agent was given as first- or second-line therapy than as third-line or greater therapy (5.0 cycles vs. 3.7 cycles respectively). The median time to treatment failure was 96 days in the prior adjuvant taxane group (range: 0–361) and 73.5 days in the no prior adjuvant taxane group (range: 0–1176).
Conclusions
This retrospective study demonstrates potential clinical activity of nab-paclitaxel in metastatic breast cancer regardless of whether patients had prior exposure to adjuvant taxanes.
Keywords: Nanoparticle, albumin-bound, nab-paclitaxel, metastatic breast cancer, adjuvant taxane
1. INTRODUCTION
Throughout the world, breast cancer is the leading cancer diagnosis in women1. In 2008, the World Health Organization estimated 1,384,155 new cases globally1. In 2012, more than 229,000 new cases were expected to be diagnosed in the United States2. Despite recent advances in diagnosis and treatment, more than 39,920 patients were expected to die from breast cancer in 2012 in the United States2.
Taxanes belong to one of the most active classes of drugs for the treatment of early and advanced stages of breast cancer3–5. Docetaxel and paclitaxel both bind to the same microtubule site, causing stabilization of the microtubules, which leads to inhibition of mitosis and tumour proliferation, and ultimately results in cell death. In early breast cancer, taxanes are routinely recommended for high-risk breast cancer, and incorporation of a taxane into an anthracycline regimen reduces not only the risks of recurrence and of breast cancer mortality, but also of overall mortality4–6. Moreover, in metastatic disease, taxanes have significant activity, translating into high response rates3.
Nanoparticle albumin-bound (nab)–paclitaxel is paclitaxel attached to albumin, allowing the microtubule stabilizer to be delivered without need of a solvent7. Nab-paclitaxel does not cause allergic reactions, and compared with polyethoxylated castor oil–based paclitaxel, it demonstrated a better response rate and improved efficacy in a phase iii randomized controlled trial. The same is also potentially true for a comparison with docetaxel, based on results from a randomized phase ii study7.
The literature is unclear about whether there is a role for re-challenge with taxanes in patients who relapse after prior adjuvant anthracycline–taxane or taxane-alone regimens8. The present study describes the clinical and pathologic characteristics of a cohort of patients with advanced breast cancer treated with nab-paclitaxel; it also assesses the clinical benefit of nab-paclitaxel therapy in women with metastatic breast cancer (mbc) previously treated with and without adjuvant taxanes in British Columbia.
2. METHODS
2.1. Study Population and Clinical Data Collection
The BC Cancer Agency (bcca) Breast Cancer Outcomes Unit (bcou) retains a prospective database of all breast cancer patients diagnosed since 1989 and referred to any of the 5 bcca regional cancer centres. Approximately 80% of all breast cancer patients in British Columbia are evaluated by a bcca physician and captured in the bcou database. The database includes detailed baseline demographics and pathology, surgical information, and adjuvant therapies including chemotherapy, hormonal therapy, biologic therapy, and radiotherapy. It also collects initial local, regional, and systemic relapse data and date and cause of death. Cancer diagnoses were based on the International Classification of Diseases for Oncology, 3rd edition.
Approval was obtained from the University of British Columbia Research Ethics Board and the bcou before the study commenced. Using the bcca Pharmacy data repository, we retrospectively identified all patients who received nab-paclitaxel at bcca, linking them with the bcou database to identify baseline demographics, pathology, and outcomes data.
Since 2007, nab-paclitaxel has been used in British Columbia in women with mbc who have progressed or relapsed on prior anthracycline-based chemotherapy or who are not medically suited to receive anthracyclines. The indications for nab-paclitaxel use include first-line treatment for patients who received anthracycline-based chemotherapy in the adjuvant setting or for whom anthracyclines are contraindicated, and second- or third-line treatment after previous combination chemotherapy with an anthracycline in a patient who has a performance status of 2 or less and a life expectancy of more than 3 months.
The standard dose of nab-paclitaxel offered in British Columbia is 260 mg/m2 every 3 weeks. Dose reduction is recommended after febrile neutropenia, sensory neuropathy, or hematologic toxicity (absolute neutrophil count of 1–1.49×109/L or platelets less than 100×109/L). In some circumstances, weekly paclitaxel was prescribed at the discretion of the oncologist, likely for tolerability reasons. Charts of patients with incomplete information (pathology, line of nab-paclitaxel therapy, dose reductions, or number of cycles) were reviewed.
2.2. Statistical Analysis
Patient and tumour characteristics; initial treatment variables such as surgery, radiation, hormonal therapy, and type of chemotherapy; cycle number and interval; and nab-paclitaxel dose reduction were summarized using descriptive statistics, including median and range. Descriptive statistics were calculated using the SPSS software application (version 14: SPSS, Chicago, IL, U.S.A.).
Outcomes of interest were first local recurrence (breast or chest wall), first regional recurrence, first distant recurrence, time to treatment failure (defined as time from the first to the last cycle of nab-paclitaxel), and proportion of patients deceased. A preplanned comparison considered the characteristics and the nab-paclitaxel treatment variables for patients who received a minimum of 1 cycle of an adjuvant taxane and for those who had no prior taxane exposure. The chi-square test was used to compare categorical variables, and p ≤ 0.05 was considered statistically significant.
3. RESULTS
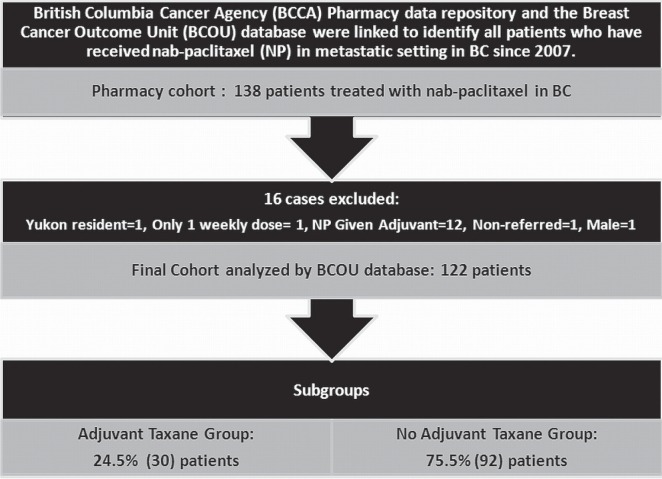
From 2007 to 2011, 138 patients in British Columbia for whom complete information was available received nab-paclitaxel. After 16 patients were excluded, 122 remained for the final study analysis (Figure 1). The reasons for exclusion included registration at the bcca, but no referral to the bcca for assessment or treatment (n = 1); male sex (n = 1); residence outside of British Columbia at the time of diagnosis (n = 1); receipt of just a single weekly dose of nab-paclitaxel (n = 1); and receipt of nab-paclitaxel in the adjuvant setting only (n = 12).
FIGURE 1.
Cohort of patients evaluated.
Most patients were positive for the estrogen receptor (77%) and negative for the human epidermal growth factor receptor 2 [her2 (87.7%)]. Slightly more than half were node-positive (58.2%), and nearly all had grade 2 or 3 tumours (86.9%). Of the 70.5% of patients who received adjuvant chemotherapy, most (64%) received an anthracycline in that setting; almost a quarter (24.6%) received an adjuvant taxane. Adjuvant hormonal therapy was given to 57.4%, and adjuvant radiotherapy to 57.4%. In this cohort, patients were more likely to have undergone a total mastectomy than breast-conserving surgery (52.5% vs. 37.7%), and 23% of patients presented with de novo metastatic (M1) disease (Table i).
TABLE I.
Tumour characteristics and initial treatment for all study patients
| Characteristic | Value [n (%)] |
|---|---|
| Patients | 122 |
| Positive nodes at early disease | |
| 0 | 30 (24.6) |
| 1–3 | 36 (29.5) |
| 4–9 | 19 (15.6) |
| 10+ | 14 (11.5) |
| Metastatic disease | |
| At presentation (M1) | 28 (23.0) |
| Distant relapse | 92 (75.4) |
| Regional relapse only | 2 (1.6) |
| Grade | |
| 1 | 9 (7.4) |
| 2 | 50 (41.0) |
| 3 | 56 (45.9) |
| Unknown | 7 (5.7) |
| Estrogen receptor statusa | |
| Positive | 94 (77.0) |
| Negative | 26 (21.3) |
| Unknown | 2 (1.6) |
| Progesterone receptor statusa | |
| Positive | 51 (41.8) |
| Negative | 48 (39.3) |
| Unknown | 23 (18.9) |
| her2 statusa | |
| Positive | 10 (8.2) |
| Negative | 107 (87.7) |
| Unknown | 5 (4.1) |
| Initial surgery | |
| None | 12 (9.8) |
| Breast-conserving | 46 (37.7) |
| Total mastectomy | 64 (52.5) |
| Initial radiotherapy | |
| None | 52 (42.6) |
| Breast and chest wall only | 23 (18.9) |
| Breast and chest wall plus regional nodes | 47 (38.5) |
| Initial hormonal therapy | |
| Yes | 70 (57.4) |
| No | 52 (42.6) |
| Initial chemotherapy | |
| None | 36 (29.5) |
| Anthracycline only | 49 (40.2) |
| Taxane only | 1 (0.8) |
| Anthracycline plus taxane | 29 (23.8) |
| Other | 7 (5.7) |
Reflects status at initial diagnosis, or if unknown at initial diagnosis, then at recurrence.
her2 = human epidermal growth factor receptor 2.
In 5 patients, her2 status was unknown (4.1%), and of the 122 patients in this cohort, only 12 received trastuzumab (Table i). Trastuzumab was given to 10 patients in the metastatic setting and to 2, adjuvantly. No patient with an unknown her2 status received trastuzumab.
Excluding patients presenting with de novo metastatic disease (M1), median time from diagnosis of invasive disease to first relapse was 3.8 years in the overall cohort. The median line of treatment in which nab-paclitaxel was delivered was second-line for the overall cohort (range: first- to eighth-line). Nab-paclitaxel was given mostly in an every-3-weeks regimen (83.6%); 14 patients (11.5%) were treated using a weekly regimen. Although the median number of nab-paclitaxel cycles prescribed for the overall cohort was 4.4 (range: 0.3–13), the median number of cycles was greater when nab-paclitaxel was given as first- or second-line therapy (median: 5 cycles vs. 3.7 cycles for third-line or greater). Approximately a quarter of all patients (25.4%) required a dose reduction of 10% or more (Table ii).
TABLE II.
Outcome and treatment characteristics for the entire study cohort
| Characteristic | Value | |
|---|---|---|
| Time from diagnosis to first relapse, non-M1 cases (years) | ||
| Median | 3.8 | |
| Range | 0.4–21.3 | |
| Nab-paclitaxel cycle [n (%)] | ||
| Weekly | 14 (11.5) | |
| Every 2 weeks | 2 (1.6) | |
| Every 3 weeks | 102 (83.6) | |
| Every 3 weeks to weekly | 4 (3.3) | |
| Cycles (n)a | ||
| Median | 4.4 | |
| Range | 0.3–13.0 | |
| First line | Median | 5.0 |
| Range | 1.0–9.0 | |
| Second line | Median | 5.0 |
| Range | 1.0–13.0 | |
| ≥Third line | Median | 3.65 |
| Range | 0.3–11.0 | |
| Line of treatment (n) | ||
| Median | 2 | |
| Range | 1–8 | |
| Time to treatment failure (days) | ||
| Median | 84 | |
| Range | 0–1176 | |
| Patients who [n (%)] | ||
| Required dose reductionb | 31 (25.4) | |
| Died | 66 (54.1) | |
1 = Each dose on an every-3-weeks schedule, every third dose on a weekly schedule, or every second dose on an every-2-weeks schedule.
Reduction of 10% or more.
The patients who received a taxane in the adjuvant setting were more likely to present with node-positive disease at diagnosis (86.7% vs. 48.9%, p = 0.007), to have grade 3 disease (70% vs. 38%, p = 0.002), and to have had initial adjuvant radiotherapy (76.7% vs. 51.1%, p < 0.001, Table iii). On the other hand, 84.8% of the patients without prior adjuvant taxane exposure were estrogen receptor–positive (compared with 53.3% of those who received a prior adjuvant taxane, p < 0.001), and consequently, adjuvant hormonal therapy was more commonly offered to those who did not have prior adjuvant taxane exposure (64.1% vs. 36.7%, p = 0.008, Table iii).
TABLE III.
Patient and tumour characteristics and initial treatment in patients receiving and not receiving an adjuvant taxane
| Characteristic |
Adjuvant taxane
|
p Valueb | |
|---|---|---|---|
| Yesa | No | ||
| Patients (n) | 30 | 92 | |
| Nodal status [n (%)] | |||
| Negative | 3 (10.0) | 27 (29.3) | |
| Positive | 26 (86.7) | 45 (48.9) | 0.007 |
| Unknown | 1 (3.3) | 20 (21.7) | |
| Positive nodes [n (%)] | |||
| 0 | 3 10.0 | 27 29.3 | 0.02c |
| 1–3 | 10 (33.3) | 26 (28.3) | |
| 4–9 | 8 (26.7) | 11 (12.0) | |
| 10+ | 7 (23.3) | 7 (7.6) | |
| Grade [n (%)] | |||
| 1 | 4 (13.3) | 5 (5.4) | |
| 2 | 5 (16.7) | 45 (48.9) | |
| 3 | 21 (70.0) | 35 (38.0) | 0.002c |
| Unknown | 0 (0.0) | 7 (7.6) | |
| her2 status [n (%)]d | |||
| Positive | 2 (6.7) | 8 (8.7) | 0.67 |
| Negative | 28 (93.3) | 79 (85.9) | |
| Unknown | 0 (0.0) | 5 (5.4) | |
| er status [n (%)]d | |||
| Positive | 16 (53.3) | 78 (84.8) | |
| Negative | 14 (46.7) | 12 (13.0) | |
| Unknown | 0 (0.0) | 2 (2.2) | |
| Initial radiotherapy [n (%)] | |||
| None | 7 (23.3) | 45 (48.9) | |
| Breast and chest wall only | 2 (6.7) | 21 (22.8) | |
| Breast and chest wall plus regional nodes | 21 (70.0) | 26 (28.3) | |
| Initial hormonal therapy [n (%)] | |||
| Yes | 11 (36.7) | 59 (64.1) | 0.008 |
| No | 19 (63.3) | 33 (35.9) | |
Includes patients who received at least 1 cycle of a taxane.
Calculated on known values only.
By exact chi-square test (when expected cell counts are not met for chi-square test in non-2×2 tables).
At initial diagnosis, or if unknown at initial diagnosis, then at recurrence.
her2 = human epidermal growth factor receptor 2; er = estrogen receptor.
Time to relapse was significantly shorter in the adjuvant taxane group than in the group without prior adjuvant taxane exposure (2.7 years vs. 4.5 years, p < 0.001), likely because the group with prior adjuvant taxane exposure had greater risk factors, such as nodal involvement, estrogen receptor–negative disease, and grade 3 tumours (Table iv).
TABLE IV.
Outcome and treatment characteristics for patients receiving and not receiving an adjuvant taxane
| Characteristic |
Adjuvant taxane
|
p Valueb | ||
|---|---|---|---|---|
| Yesa | No | |||
| Patients (n) | 30 | 92 | ||
| Time from diagnosis to first relapse, non-M1 cases only (years) | ||||
| Median | 2.7 | 4.5 | <0.001 | |
| Range | 0.8–6.4 | 0.4–21.3 | ||
| Cycles (n) | ||||
| Median | 4.2 | 4.6 | 0.71 | |
| Range | 0.3–13.0 | 1.0–11.0 | ||
| Line of treatment (n) | ||||
| Median | 3 | 2 | 0.27 | |
| Range | 1–7 | 1–8 | ||
| Nab-paclitaxel in | ||||
| First line | Patients (n) | 5 | 23 | |
| Median cycles (n) | 5.3 | 5 | 0.98 | |
| Range cycles (n) | 2–6 | 1–9 | ||
| Second line | Patients (n) | 9 | 31 | |
| Median cycles (n) | 8 | 5 | 0.02 | |
| Range cycles (n) | 1–13 | 1–9 | ||
| ≥Third line | Patients (n) | 16 | 38 | |
| Median cycles (n) | 3.15 | 4 | ||
| Range cycles (n) | 0.3–6.0 | 1–11 | 0.32 | |
| Time to treatment failure (days) | ||||
| Median | 96 | 73.5 | 0.58 | |
| Range | 0–361 | 0–1176 | ||
| Patients who [n(%)] | ||||
| Required dose reductionc | 6 (20.0) | 25 (27.2) | 0.43 | |
| Died | 22 (73.3) | 44 (47.8) | 0.02 | |
Includes patients who received at least 1 cycle of a taxane.
Calculated on known values only.
Reduction of 10% or more.
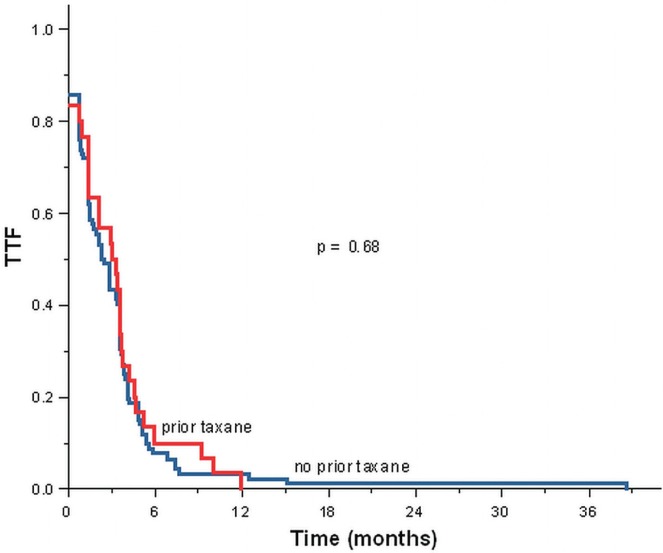
Median time to treatment failure was 84 days for the overall cohort. We observed no apparent statistically significant difference in the median time to treatment failure for the group with prior adjuvant taxane exposure compared with the non-exposure group (96 days vs. 73.5 days, p = 0.58, Table iv, Figure 2).
FIGURE 2.
Time to treatment failure (ttf), first prescription date for nab-paclitaxel to last prescription date for nab-paclitaxel.
Similar dose reduction rates were observed between the adjuvant taxane and the no adjuvant taxane groups (20.0% vs. 27.2%, p = 0.43). Overall, 54% of the study cohort had died at the time of writing, with a greater proportion of deaths in the adjuvant taxane group (73.3%) compared with the no adjuvant taxane group (47.8%, p = 0.02, Table iv).
4. DISCUSSION
The taxane drug class—which includes docetaxel and paclitaxel—contains some of the most active therapeutic agents in the treatment of breast cancer3–5. These cytotoxic antimicrotubule agents inhibit the de-polymerization of tubulin in the spindle apparatus, subsequently leading to defects in mitotic spindle assembly, chromosome segregation, and cell division—ultimately resulting in cell death9. Nab-paclitaxel is a solvent-free albumin-bound formulation of paclitaxel that was developed to avoid the toxicities of the polyethoxylated castor oil vehicle used in solvent-based paclitaxel and to potentially deliver paclitaxel more selectively to tumours7.
In the pivotal phase iii trial of nab-paclitaxel 260 mg/m2 compared with polyethoxylated castor oil–based paclitaxel 175 mg/m2 every 3 weeks in the treatment of mbc, the response rate was greater (33% vs. 19%, p = 0.001) and the time to progression longer (23.0 weeks vs. 16.9 weeks; hazard ratio: 0.75; p = 0.006) in favour of nab-paclitaxel7. The incidence of grade 4 neutropenia was also significantly lower for patients treated with nab-paclitaxel than with solvent-based paclitaxel (9% vs. 22%, p = 0.001), but grade 3 sensory neuropathy was more common in the nab-paclitaxel group (10% vs. 2%, p = 0.001)7.
In a subsequent randomized phase ii trial, the antitumour activity and safety of weekly and every-3-weeks nab-paclitaxel were compared with docetaxel as first-line treatment in patients with mbc10. Progression-free survival was significantly longer with nab-paclitaxel 150 mg/m2 weekly than with docetaxel 100 mg/m2 every 3 weeks, as determined by investigator assessment (14.6 months vs. 7.8 months, p = 0.01)10. In terms of toxicity, the incidence of sensory neuropathy was higher in the nab-paclitaxel cohort than in the standard paclitaxel group10. However, this adverse side effect rapidly resolved after treatment interruption and dose reduction. Conversely, neutropenia and febrile neutropenia were significantly more frequent in the docetaxel arm (8% vs. 1%)10.
Recently, the Cancer and Leukemia Group B presented results of their 40502 trial comparing weekly paclitaxel (90 mg/m2), nab-paclitaxel (150 mg/m2), and ixabepilone (16 mg/m2) with bevacizumab (10 mg/kg every 2 weeks) as first-line therapy for locally recurrent or mbc11. A total of 799 patients were enrolled, and with a median follow-up of 12 months, analysis of progression-free survival failed to demonstrate superiority for either experimental arm compared with paclitaxel: 10.6 months with paclitaxel, 9.2 months with nab-paclitaxel, and 7.6 months with ixabepilone, with hazard ratios (95% confidence intervals) of 0.94 (0.73 to 1.22) and 0.66 (0.51 to 0.84) for paclitaxel relative to nab-paclitaxel and ixabepilone respectively11. Hematologic toxicities and sensory neuropathy were also greater in the nab-paclitaxel arm than in the paclitaxel-alone arm11.
The literature contains very few data about nab-paclitaxel re-challenge. Blum et al.8 analyzed 181 patients in an open-label phase ii trial of nab-paclitaxel; 100 patients received paclitaxel or docetaxel in the adjuvant or metastatic setting. Response rates were 14% and 16% for 100 mg/m2 and 125 mg/m2 weekly nab-paclitaxel respectively, with 12% and 21% of patients having stable disease8. For patients who had received both taxanes as prior therapy, the response rate was only 7%8.
Nab-paclitaxel appears to have a more preferable toxicity profile compared with docetaxel and paclitaxel. Nab-paclitaxel has been shown to be associated with fewer grade 3 and 4 toxicities, including allergic reactions, neutropenia, and febrile neutropenia7,10. Although nab-paclitaxel has the highest drug cost per cycle compared with docetaxel and paclitaxel, an economic analysis that included utilization of health care resources for the delivery of chemotherapy and the management of grades 3 and 4 toxicity showed that nab-paclitaxel was a more economic option than docetaxel12. For those reasons, nab-paclitaxel might be considered a reasonable taxane of choice for the treatment of mbc, particularly in patients with prior exposure to an adjuvant solvent-based taxane.
British Columbia is one of the few provinces in Canada that has open access to a choice of taxanes (nab-paclitaxel, docetaxel, paclitaxel) for the treatment of mbc. Our study describes the use of nab-paclitaxel in a general population with mbc, but also compares re-challenge using a taxane in patients who had and had not received prior adjuvant taxane treatment. Time to treatment failure, dose reductions, median number of cycles received, and total number of cycles received were similar whether patients had prior adjuvant taxane exposure or not. Those findings potentially support the hypothesis of non-cross-resistance for nab-paclitaxel despite exposure to the other taxanes currently used in the treatment of breast cancer. As well, our results are consistent with other studies of palliative treatment, in which higher response rates are typically observed in first- and second-line treatment rather than in later lines of therapy.
Our study is not without limitations. The cohort evaluated was modest in size, and the data were collected retrospectively. On the other hand, our cohort represents routine clinical practice in that it includes patients who received multiple lines of treatment (median: 2; range: 1–8) and who were not participants in randomized clinical trials. That feature of our study enhances its generalizability.
5. CONCLUSIONS
With the greater use of adjuvant taxanes in current practice, there will be a need for additional agents with clinical activity in patients who unfortunately experience a relapse. In our retrospective study, the use of nab-paclitaxel for recurrent disease showed clinical benefit regardless of prior adjuvant exposure (docetaxel or paclitaxel) and supports access to nab-paclitaxel for this indication. Future work will be needed to identify more-efficacious nab-paclitaxel combinations and predictive biomarkers for its more optimal use, and for cost effectiveness of this agent in the treatment of mbc.
6. CONFLICT OF INTEREST DISCLOSURES
The authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.World Health Organization, International Agency for Research on Cancer (iarc) Breast Cancer Incidence, Mortality and Prevalence Worldwide in 2008: Summary [Web resource] Lyon, France: IARC; 2008. [Available at: http://globocan.iarc.fr (under Fact Sheets, choose “Breast” and “World”); cited February 3, 2013] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Piccart–Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–6. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 4.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel, but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–83. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Gelderblom H, Verweij J, Nooter K, Sparreboom A. Cremophor EL: the drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–8. doi: 10.1016/S0959-8049(01)00171-X. [DOI] [PubMed] [Google Scholar]
- 7.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase iii trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil–based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 8.Blum JL, Savin MA, Edelman G, et al. Phase ii study of weekly albumin-bound paclitaxel for patients with metastatic breast cancer heavily pretreated with taxanes. Clin Breast Cancer. 2007;7:850–6. doi: 10.3816/CBC.2007.n.049. [DOI] [PubMed] [Google Scholar]
- 9.Rowinsky EK, Donehower RC. Paclitaxel (Taxol) N Engl J Med. 1995;332:1004–14. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 10.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–19. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 11.Rugo HS, Barry WT, Moreno–Aspitia A, et al. calgb 40502/ncctg N063H: randomized phase iii trial of weekly paclitaxel (p) compared to weekly nanoparticle albumin bound nab-paclitaxel (np) or ixabepilone (ix) with or without bevacizumab (b) as first-line therapy for locally recurrent or metastatic breast cancer (mbc). [abstract CRA1002] J Clin Oncol. 2012. p. 30. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=99475; cited January 31, 2013] [DOI] [PMC free article] [PubMed]
- 12.Dranitsaris G, Cottrell W, Spirovski B, Hopkins S. Economic analysis of albumin-bound paclitaxel for the treatment of metastatic breast cancer. J Oncol Pharm Pract. 2009;15:67–78. doi: 10.1177/1078155208098584. [DOI] [PubMed] [Google Scholar]