Abstract
Background
Panitumumab is a fully human monoclonal antibody, directed against the epidermal growth factor receptor, that was shown to be effective in third-line metastatic colorectal cancer. We performed a retrospective analysis of patients with chemo-refractory non-KRAS-mutated metastatic colorectal cancer, who received panitumumab at the Jewish General Hospital in Montreal, Canada, between 2009 and 2012.
Methods
This chart review included 44 patients (median age: 60 years; performance status: 0–3), of whom 50% had already received three lines of treatment. The primary endpoint was progression-free survival (pfs). Secondary endpoints were overall survival and safety. Tumour progression was determined by radiologic assessments performed once every 3 months per clinical guidelines or by clinical deterioration as determined by the clinician–investigator.
Results
In our sample, median pfs was 21.86 ± 5.23 weeks (95% confidence interval: 12.9 to 36.9 weeks) and overall survival was 35.14 ± 7.75 weeks (95% confidence interval: 25.6 to 73.4 weeks) with a median of 5 cycles of panitumumab treatment. The most frequently reported toxicities with panitumumab were skin toxicity (16.2% grade 3) and hypomagnesemia (10.8% grade 3). No infusion reactions were reported.
Conclusions
Despite a small sample size from a single institution, our survival and efficacy data are encouraging and comparable to results obtained from the registration panitumumab trial. Our findings suggest that panitumumab can be effective and tolerable in a real-world setting.
Keywords: Metastatic colorectal cancer, panitumumab, progression-free survival, overall survival, safety
1. INTRODUCTION
Panitumumab (Vectibix: Amgen, Thousand Oaks, CA, U.S.A.) is a fully human monoclonal antibody directed against the epidermal growth factor receptor (egfr)1–4. Panitumumab binds to egfr and prevents receptor dimerization, autophosphorylation, and activation of downstream signalling pathways responsible for cellular proliferation and tumour growth5,6. The drug has been studied as monotherapy and in combination with irinotecan, oxaliplatin, and 5-fluorouracil–based chemotherapy in various lines of metastatic colorectal cancer (mcrc) treatment7,8.
In third-line treatment, cetuximab, a chimeric monoclonal anti-egfr antibody was shown to be active and well tolerated in colorectal cancer patients9–14. To evaluate the effectiveness of panitumumab, a randomized phase iii trial was designed in which 463 patients were randomly assigned to the study drug (6 mg/kg every 2 weeks) plus best supportive care (bsc) or to bsc alone. Results showed a statistically significant and clinically meaningful improvement in mean progression-free survival (pfs) in the group receiving panitumumab compared with the group receiving bsc alone (13.8 weeks vs. 8.5 weeks, p < 0.0001). However, no difference in overall survival (os) was observed, a finding that was attributed to the effects of panitumumab in the bsc group after the preplanned crossover7.
Recently, the predictive role of KRAS was explored in patients receiving anti-egfr therapies15–22. Results showed that 35%–45% of patients with mcrc harbour mutations in the KRAS gene and do not benefit from anti-egfr therapies. A follow-up study conducted by Amado et al.22 showed that the effect of panitumumab on pfs was significantly greater in patients with wild-type KRAS than in those with the mutated gene (p < 0.0001). Overall survival was also longer in patients with wild-type KRAS [hazard ratio: 0.67; 95% confidence interval (ci): 0.55 to 0.82]. As a result of those studies, KRAS status is now required before initiating anti-egfr treatment.
We performed a retrospective chart review and examined pfs, os, and safety in mcrc patients with non-mutated KRAS, who failed all lines of chemotherapy and who received panitumumab as palliative treatment at the Segal Cancer Centre of the Jewish General Hospital in Montreal, Quebec, Canada.
2. METHODS
2.1. Patient Population
Patients more than 18 years of age with mcrc and documented evidence of failure of fluoropyrimidines, oxaliplatin, and irinotecan were eligible for panitumumab treatment between July 2009 and March 2012. They received either panitumumab 6 mg/kg every 2 weeks or the same dose of panitumumab plus irinotecan 180 mg/m2 according to the treating physician’s choice until disease progression or unacceptable toxicity. Treatment with panitumumab with or without irinotecan was defined as receiving at least 1 infusion. In these patients, KRAS status was determined by polymerase chain reaction and sequencing at the Jewish General Hospital. Patients who had a mutated KRAS gene or who had previously been treated with cetuximab were excluded from the study. Because the present review was intended as a good clinical practice report, it did not require submission to the Research Ethics Board.
2.2. Statistical Analysis
The primary endpoint of the study was pfs, defined as the interval from treatment assignment to progression or death. Two survival outcomes were recorded:
-
Tumour progression
Progression was determined using the Response Evaluation Criteria in Solid Tumors (version 1.1) or clinical deterioration as documented by the treating clinician. Patients underwent computed tomography imaging every 3 months per standard clinical guidelines for tumour assessments or to confirm suspected clinical deterioration (clinician’s choice). Tumour response was assessed by the treating physician, who documented it in the medical chart.
-
Death
Date of death was captured from patient charts.
Secondary endpoints included os and safety. Adverse events attributable to panitumumab treatment were reported and graded by the treating physician in the chart during a medical visit. The grading of toxicities was based on safety guidelines per the Common Terminology Criteria for Adverse Events, version 4.0.
The statistical analysis was performed using the Strata 10 software application (StataCorp LP, College Station, TX, U.S.A.). The survival analysis used the Kaplan–Meier method23.
3. RESULTS
3.1. Patients and Treatments
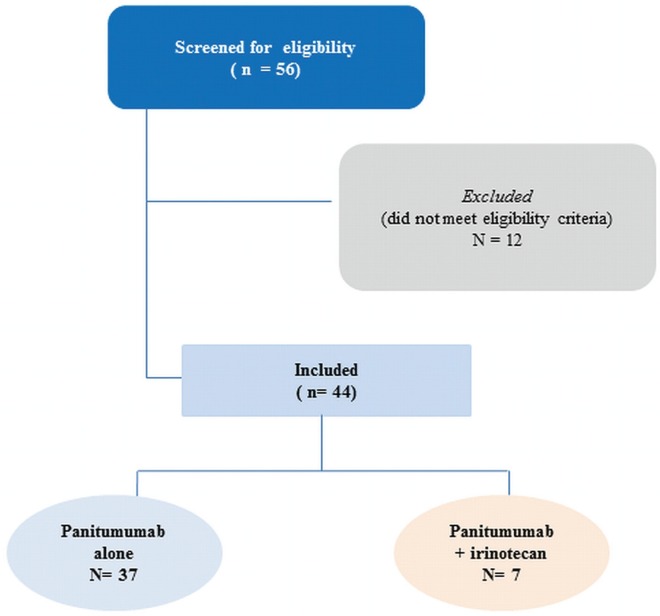
As shown in Figure 1, 56 patients had a wild-type KRAS gene, and 44 received at least 1 infusion of panitumumab with or without irinotecan. Of the 44 patients, 7 were treated with panitumumab and irinotecan (physician’s choice in clinical practice), and 37, with panitumumab alone.
FIGURE 1.
consort diagram for the study.
In the group receiving panitumumab alone, median age was 60 years (range: 41–82 years). Performance status [Eastern Cooperative Oncology Group (ecog)] varied: 24% were scored 0; 54%, 1; 16%, 2; and 3%, 3 (Table i). All patients had received at least 2 prior lines of chemotherapy; 51.4% (n = 19) had received 3 prior lines of chemotherapy, and 8.1% (n = 3) had received 4 prior lines of chemotherapy (Table i).
Table I.
Baseline characteristics of patients receiving panitumumab
| Characteristic | Value |
|---|---|
| Patients (n) | 37 |
| Age (years)a | |
| Mean | 60±10.6 |
| Range | 42–81 |
| Age ≥65 years (n) | 12 |
| ecog performance status [n (%)] | |
| 0 | 9 (24.3) |
| 1 | 20 (54) |
| 2 | 6 (16.2) |
| 3 | 1 (2.7) |
| Prior lines of chemotherapy [n (%)] | |
| 1b | 37 (100) |
| 2c | 37 (100) |
| 3d | 19 (51.4) |
| 4e | 3 (8.1) |
At treatment start.
folfox or folfiri ± bevacizumab; capecitabine ± oxaliplatin; 5-fluorouracil–leucovorin.
folfox or folfiri ± bevacizumab; irinotecan; capecitabine.
Oxaliplatin; irox (irinotecan–oxaliplatin); folfiri; irinotecan.
folfox.
ecog = Eastern Cooperative Oncology Group.
Table ii shows the exposure of these patients to panitumumab treatment: 40.5% (n = 15) had less than 3 months’ exposure (1–5 infusions); 32.4% (n = 12) had 3–6 months’ exposure (6–12 infusions); 21.6% (n = 8) had 6–11.5 months’ exposure (14–23 infusions), and 5.4% (n = 2) had more than 14.5 months’ exposure (29 infusions). The median number of infusions was 5. Treatment was discontinued upon disease progression. No patient experienced an adverse event severe enough to require treatment discontinuation.
TABLE II.
Treatment exposure to panitumumab alone
| Exposure | Value [n (%)] |
|---|---|
| Patients | 37 |
| Infusions | |
| 1 | 2 |
| 2+ | 2 |
| 3+ | 4 |
| 4 | 2 |
| 5 | 5 |
| 6+ | 3 |
| 7 | 1 |
| 8 | 2 |
| 9+ | 2 |
| 11 | 1 |
| 12+ | 3 |
| 14 | 2 |
| 16 | 1 |
| 18 | 1 |
| 19 | 1 |
| 20 | 1 |
| 21 | 1 |
| 23+ | 1 |
| 29+ | 2 |
| Treatment exposure | |
| <3 Months (1–5 infusions) | 15 (40.5) |
| 3 to 6 Months (6–12 infusions) | 12 (32.4) |
| >6 to 11.5 Months (14–23 infusions) | 8 (21.6) |
| 14.5 Months (29 infusions) | 2 (5.4) |
| Median cycles (n) | 5 |
3.2. Efficacy
We conducted two subanalyses. The first included all 44 patients treated with panitumumab with or without irinotecan. The second included the 37 patients treated with panitumumab alone.
3.2.1. PFS
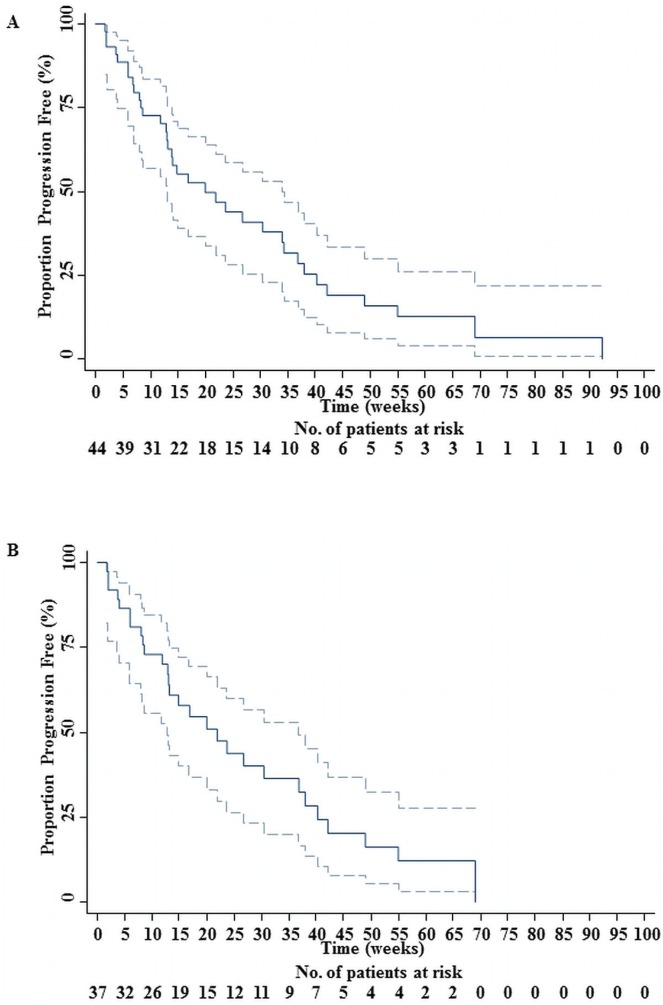
In the overall group (44 patients), 35 progressions (79.55%) and 9 censored observations (20.45%) were recorded. Median pfs was 20 weeks, with a standard error of 5.8 weeks (95% ci: 13 to 34 weeks). Mean pfs was 28.48 weeks, with a standard error of 4.11 weeks (95% ci: 20.44 to 36.53 weeks). The estimated progression rate of the population was 0.034 new progressions per week (95% ci: 0.024 to 0.047 new progressions per week). Figure 2(A) shows the associated Kaplan–Meyer pfs curve.
FIGURE 2.
(A) Progression-free survival (pfs) for 44 patients receiving irinotecan or panitumumab, or both. The solid line represents the pfs curve for patients treated at the Jewish General Hospital. (B) The pfs for 37 patients receiving panitumumab alone. The solid line represents the pfs curve for patients treated at the Jewish General Hospital. The dotted lines mark the 95% confidence limits.
In the group receiving panitumumab alone (37 patients), 28 progressions (75.68%) and 9 censored observations (24.32%) were registered. Median pfs was 21.86 weeks (Amado et al. reported a median pfs of 12.3 weeks for panitumumab), with a standard error of 5.23 weeks (95% ci: 12.86 to 36.86 weeks). Mean pfs was 27.34 weeks, with a standard error of 3.82 weeks (95% ci: 19.85 to 34.82 weeks). The estimated progression rate of this group was 0.0340 new progressions per week (95% ci: 0.0234 to 0.0492 new progressions per week). Figure 2(B) shows the associated Kaplan–Meier pfs curve.
The addition of irinotecan did not seem to improve pfs [Figure 2(A)].
3.2.2. OS
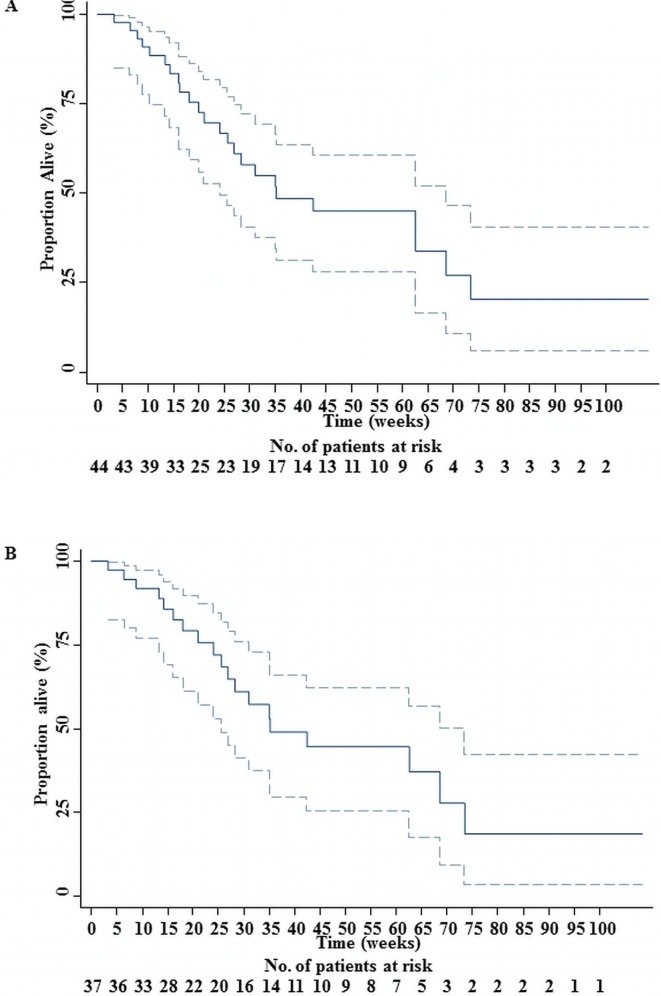
In the overall group (44 patients), 24 deaths (54.55%) and 20 censored observations (45.45%) were reported. Median os was 35.14 weeks, with a standard error of 9.24 weeks (95% ci: 24 to 68.57 weeks). The restricted mean os was 50.36 weeks, with a standard error of 6.50 weeks (95% ci: 37.62 to 63.11 weeks). The restricted mean os is censored because of the analysis time, but the extended mean os can be estimated up to 64.09 weeks. The death rate for the population was estimated at 0.0158 deaths per week (95% ci: 0.0106 to 0.0236 deaths per week). Figure 3(A) shows the associated Kaplan–Meier survival curve.
FIGURE 3.
(A) Overall survival (os) for 44 patients receiving panitumumab with or without irinotecan. The solid line represents the os curve of patients treated at the Jewish General Hospital (B) Overall survival (os) for 37 patients receiving panitumumab alone. The solid line represents the os curve of patients treated at the Jewish General Hospital. The dotted lines mark the 95% confidence limits.
In the group receiving panitumumab alone (37 patients), 19 deaths (51.35%) and 18 censored observations (48.65%) were recorded. Median os was 35.14 weeks (Amado et al. reported a median os of 8.1 months for panitumumab), with a standard error of 7.75 weeks (95% ci: 25.57 to 73.43 weeks). The restricted mean was 50.96 weeks, with a standard error of 7.12 weeks (95% ci: 37.00 to 64.91 weeks). Once again, the extended mean os can be estimated as 62.91 weeks. The estimated death rate of this group was 0.0152 deaths per week (95% ci: 0.0097 to 0.0239 deaths per week). Figure 3(B) shows the associated Kaplan–Meier survival curve.
3.2.3. Safety
Table iii lists 11 adverse events investigated in the 37 patients treated with panitumumab alone (clinicians are more likely to associate these symptoms with disease progression, pain, and opiates). The most frequently noted adverse events were skin toxicities (n = 21, 57%) and hypomagnesemia (n = 15, 41%). Grade 3 skin toxicities and hypomagnesemia occurred in 6 patients (16%) and 4 patients (11%) respectively. A grade 3 paronychia was registered in 1 patient (2.7%).
TABLE III.
Safety profile related to panitumumab treatment
| Adverse event |
Patients experiencing [n of 37 (%)]
|
|
|---|---|---|
| Any grade | Grade 3 | |
| Skin or integument toxicity | 21 (56.8) | 6 (16.2) |
| Eye toxicity | 1 (2.7) | 0 (0) |
| Paronychia | 2 (5.4) | 1 (2.7) |
| Abdominal pain | 2 (5.4) | 0 (0) |
| Constipation | 2 (5.4) | 0 (0) |
| Diarrhea | 3 (8.1) | 0 (0) |
| Vomiting | 1 (2.7) | 0 (0) |
| Fatigue and general deterioration | 12 (32.4) | 12 (32.4) |
| Hypomagnesemia | 15 (40.5) | 4 (10.8) |
The deaths that occurred among the patients in the panitumumab-only group (n = 20, 54%) were all deemed to be a result of disease progression and not of treatment. Disease progression was determined by clinical deterioration (for example, disabling fatigue) in 12 patients treated with panitumumab alone (32%) and by the once-every-3-months radiologic assessment in 25 patients (68%).
4. DISCUSSION
In this retrospective study, we collected and analyzed the clinical outcomes of treatment with panitumumab in patients with wild-type KRAS and progressive mcrc, in whom at least two lines of chemotherapy failed. In the initial study demonstrating the requirement of wild-type KRAS for panitumumab efficacy in third-line treatment for mcrc22, the median age of the patients was 62.5 years, and most patients had a performance status score between 0 (53%) and 1 (56%). Although the age range in our patients was similar, performance status showed more variability, with ecog scores spanning the entire 0–3 range (most patients had an ecog score between 1 and 2), likely because our data were collected in a real-world clinical setting rather than in a clinical trial. Our patients had received at least two previous lines of treatment, and in 59.5% of them, panitumumab was used as the fourth or fifth line.
Taken together, the foregoing observations suggest that our patients were performing more poorly than those randomized in the clinical trial conducted by Amado and colleagues22. Perhaps as a consequence, our patients received a median of 5 panitumumab infusions compared with the median of 8 infusions in the Amado et al. trial. Despite a lower number of infusions for our population, our patients achieved a pfs of 21.86 ± 5.23 weeks (95% ci: 12.9 to 36.9 weeks), which is encouraging compared with the published median pfs of 12.3 weeks in the 2008 study22. However, that difference in pfs might also be a result of differences in the assessment of disease progression in clinical practice (imaging frequency or confirmation, clinician assessment) compared with that in a clinical trial setting. Indeed, the os that we observed was similar to the os in the registration trial (8.78 months vs. 8.1 months)7, which is consistent with the idea that pfs is not the best predictor of os in the heavily pretreated mcrc setting.
We therefore conclude that median pfs with panitumumab treatment looks favourable compared with the pfs documented in a previous report22. We observed no differences in os or safety despite the small size of our population. Also, there appeared to be no differences in efficacy between panitumumab alone and panitumumab plus irinotecan. That observation suggests that panitumumab alone may be the most reasonable choice in this setting, although the small sample size warrants caution in the interpretation.
Interestingly, a French group presented data about the use of panitumumab as a single agent in 269 mcrc patients in real-world practice24. Median os was 6.71 months, which is shorter than that previously described (Amado et al.: 8.1 months). The authors attribute the difference to the status of the study as a real-world report and not as a clinical trial enrolling younger patients with better health states.
The most frequently reported toxicities related to panitumumab treatment were skin toxicity and hypomagnesemia. Only grade 3 skin toxicity and hypomagnesemia were reported for both the present study and other published studies7,22. Given the small sample size, we could not obtain convincing survival data stratified by the presence or absence of skin reaction, or a lower compared with a normal serum magnesium. Higher grade 3 hypomagnesemia was noted in our setting, probably because our population was heavily pre-treated compared with the patients in the clinical trials. Moreover, the other 9 reported adverse events (eye toxicity, paronychia, anorexia, abdominal pain, constipation, diarrhea, vomiting, fatigue, and general physical deterioration) were less frequent in our study than in other reported studies involving panitumumab treatment7,22. No infusion reactions were reported in our setting.
5. CONCLUSIONS
We found that the pfs and os attained with panitumumab therapy in our setting—heavily pre-treated mcrc patients (>50% with 3+ lines, 8% with 4+ lines) with poorer performance status than is typically permitted in clinical trials—were in line with previously published reports7,22.
Because real-world data are often required by regional authorities to approve and reimburse medication, we believe that our results are encouraging and that our data may facilitate access by patients to this oncology medication in a real-world setting. Nevertheless, analysis of a larger sample of patients with mcrc is warranted to assess the proper per label use in regular clinical practice.
6. CONFLICT OF INTEREST DISCLOSURES
All authors declare no financial conflicts of interest.
7. REFERENCES
- 1.Foon KA, Yang XD, Weiner LM, et al. Preclinical and clinical evaluations of abx-egf, a fully human anti-epidermal growth factor receptor antibody. Int J Radiat Oncol Biol Phys. 2004;58:984–90. doi: 10.1016/j.ijrobp.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 2.Rowinsky EK, Schwartz GH, Gollob JA, et al. Safety, pharmacokinetics, and activity of abx-egf, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer. J Clin Oncol. 2004;22:3003–15. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase ii trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 4.Yang XD, Jia XC, Corvalan JR, Wang P, Davis CG, Jakobovits A. Eradication of established tumors by a fully human monoclonal antibody to the epidermal growth factor receptor without concomitant chemotherapy. Cancer Res. 1999;59:1236–43. [PubMed] [Google Scholar]
- 5.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–85. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NE, Lane HA. erbb receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [Erratum in: Nat Rev Cancer 2005;5:580] [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Peeters M, Siena S, et al. Open-label phase iii trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy- refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 8.Peeters M, Price TJ, Cervantes A, et al. Randomized phase iii study of panitumumab with fluorouracil, leucovorin, and irinotecan (folfiri) compared with folfiri alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706–13. doi: 10.1200/JCO.2009.27.6055. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 10.Jonker DJ, O’Callaghan CJ, Karapetis CS, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 11.Wilke H, Glynne–Jones R, Thaler J, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: mabel study. J Clin Oncol. 2008;26:5335–43. doi: 10.1200/JCO.2008.16.3758. [DOI] [PubMed] [Google Scholar]
- 12.Sobrero AF, Maurel J, Fehrenbacher L, et al. epic: phase iii trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311–19. doi: 10.1200/JCO.2007.13.1193. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 14.Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–71. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 15.Benvenuti S, Sartore–Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the Ras/Raf signaling pathway impairs the response of metastatic colorectal cancers to antiepidermal growth factor receptor antibody therapies. Cancer Res. 2007;67:2643–8. doi: 10.1158/0008-5472.CAN-06-4158. [DOI] [PubMed] [Google Scholar]
- 16.Di Fiore F, Blanchard F, Charbonnier F, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Roock W, Piessevaux H, De Schutter J, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 18.Freeman DJ, Juan T, Reiner M, et al. Association of K-ras mutational status and clinical outcomes in patients with metastatic colorectal cancer receiving panitumumab alone. Clin Colorectal Cancer. 2008;7:184–90. doi: 10.3816/CCC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 19.Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 20.Karapetis CS, Khambata–Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 21.Jimeno A, Messersmith WA, Hirsch FR, Franklin WA, Eckhardt SG. KRAS mutations and sensitivity to epidermal growth factor receptor inhibitors in colorectal cancer: practical application of patient selection. J Clin Oncol. 2009;27:1130–6. doi: 10.1200/JCO.2008.19.8168. [DOI] [PubMed] [Google Scholar]
- 22.Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 23.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York, NY: Springer Science and Business Media; 2003. [Google Scholar]
- 23.Grude F, Ramée JF, Guivarch L, et al. Panitumumab as a single agent in 269 metastatic colorectal cancer patients in the real practice: a post ema approval study. J Clin Oncol. 2012;30 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=114&abstractID=99969; cited January 31, 2013] [Google Scholar]