TABLE I.
Selected trials in the first-line setting
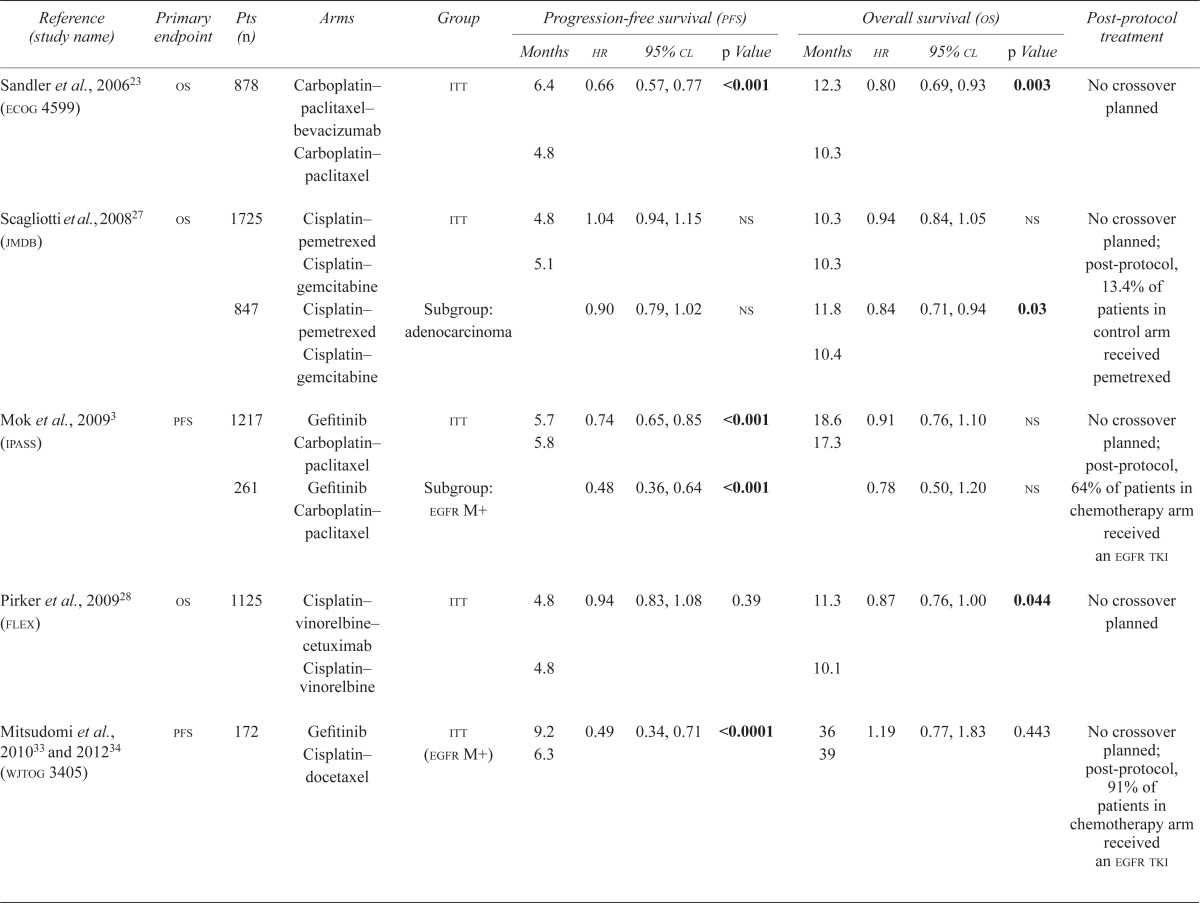
| Reference (study name) | Primary endpoint | Pts ( n ) | Arms | Group | Progression-free survival (pfs) | Overall survival (os) | Post-protocol treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Months | hr | 95% cl | p Value | Months | hr | 95% cl | p Value | ||||||
| Sandler et al., 200623 (ecog 4599) | os | 878 | Carboplatin–paclitaxel–bevacizumab | itt | 6.4 | 0.66 | 0.57, 0.77 | <0.001 | 12.3 | 0.80 | 0.69, 0.93 | 0.003 | No crossover planned |
| Carboplatin–paclitaxel | 4.8 | 10.3 | |||||||||||
| Scagliotti et al., 200827 (jmdb) | os | 1725 | Cisplatin–pemetrexed | itt | 4.8 | 1.04 | 0.94, 1.15 | ns | 10.3 | 0.94 | 0.84, 1.05 | ns | No crossover planned; post-protocol, 13.4% of patients in control arm received pemetrexed |
| Cisplatin–gemcitabine | 5.1 | 10.3 | |||||||||||
| 847 | Cisplatin–pemetrexed | Subgroup: adenocarcinoma | 0.90 | 0.79, 1.02 | ns | 11.8 | 0.84 | 0.71, 0.94 | 0.03 | ||||
| Cisplatin–gemcitabine | 10.4 | ||||||||||||
| Mok et al., 20093 (ipass) | pfs | 1217 | Gefitinib Carboplatin–paclitaxel | itt | 5.7 | 0.74 | 0.65, 0.85 | <0.001 | 18.6 | 0.91 | 0.76, 1.10 | ns | No crossover planned; post-protocol, 64% of patients in chemotherapy arm received an egfr tki |
| 5.8 | 17.3 | ||||||||||||
| 261 | Gefitinib Carboplatin–paclitaxel | Subgroup: egfr M+ | 0.48 | 0.36, 0.64 | <0.001 | 0.78 | 0.50, 1.20 | ns | |||||
| Pirker et al., 200928 (flex) | os | 1125 | Cisplatin–vinorelbine–cetuximab | itt | 4.8 | 0.94 | 0.83, 1.08 | 0.39 | 11.3 | 0.87 | 0.76, 1.00 | 0.044 | No crossover planned |
| Cisplatin–vinorelbine | 4.8 | 10.1 | |||||||||||
| Mitsudomi et al., 201033 and 201234 (wjtog 3405) | pfs | 172 | Gefitinib Cisplatin–docetaxel | itt (egfr M+) | 9.2 | 0.49 | 0.34, 0.71 | <0.0001 | 36 | 1.19 | 0.77, 1.83 | 0.443 | No crossover planned; post-protocol, 91% of patients in chemotherapy arm received an egfr tki |
| 6.3 | 39 | ||||||||||||
| Reck et al., 201025 (avail) | pfs (originally os) | 692 | Cisplatin–gemcitabine–bevacizumab | itt (7.5 mg/kg bevacizumab) | 6.7 | 0.75 | 0.64, 087 | 0.0003 | 13.6 | 0.93 | 0.78, 1.11 | 0.42 | Crossover not permitted |
| Cisplatin–gemcitabine | 6.1 | 13.1 | |||||||||||
| 698 | Cisplatin–gemcitabine–bevacizumab | itt (15 mg/kg bevacizumab) | 6.5 | 0.85 | 0.73, 1.00 | 0.0456 | 13.4 | 1.03 | 0.86, 1.23 | 0.761 | |||
| Cisplatin–gemcitabine | 6.1 | 13.1 | |||||||||||
| Zhou et al., 201130 (optimal) | pfs | 154 | Erlotinib Carboplatin–gemcitabine | Received 1 dose of drug (egfr M+) | 13.1 | 0.16 | 0.10, 0.26 | <0.0001 | 22.7 | 1.065 | ns | No crossover planned; post-protocol, 71% of patients in chemotherapy arm received an egfr tki. | |
| 4.6 | 28.9 | ||||||||||||
| Han et al., 201231 (First-signal) | os | 309 | Gefitinib Cisplatin–gemcitabine | itt | 5.8 | 1.2 | 0.94, 1.5 | ns | 22.3 | 0.932 | 0.72, 1.2 | 0.604 | No crossover planned; post protocol, 75% of in patients chemotherapy arm received an egfr tki |
| 6.4 | 22.9 | ||||||||||||
| 96 | Gefitinib Cisplatin–gemcitabine | Subgroup: egfr M+ | 0.38 | 0.21, 0.68 | <0.001 | 1.043 | 0.50, 2.2 | ns | |||||
| Rosell et al., 201232 (eurtac) | pfs | 173 | Erlotinib Standard chemotherapy | itt (egfr M+) | 9.7 | 0.37 | 0.25, 0.54 | <0.0001 | 19.3 | 1.04 | 0.65, 1.68 | 0.87 | Crossover |
| 5.2 | 19.5 | ||||||||||||
| Yang et al., 201235 (lux-Lung3) | pfs | 345 | Afatinib Cisplatin–pemetrexed | itt (egfr M+) | 11.1 | 0.58 | 0.43, 0.78 | 0.0004 | No crossover planned | ||||
| 6.9 | |||||||||||||
Pts = patients; hr = hazard ratio; cl = confidence limits; ecog = Eastern Cooperative Oncology Group; itt = intention-to-treat; ns = nonsignificant; egfr = epidermal growth factor receptor; M+ = mutation positive; tki = tyrosine kinase inhibitor.
