Abstract
Comparative effectiveness research has become an integral part of health care planning in most developed countries. In a simulated cohort of women, aged 30–65, who tested positive for BRCA1 or BRCA2 mutations, we compared outcomes of mammography with and without MRI, prophylactic oophorectomy with and without mastectomy, mastectomy alone, and chemoprevention. Methods: Using Treeage 9.02 software, we developed Markov models with 25,000 Monte Carlo simulations and conducted probabilistic sensitivity analysis. We based mutation penetrance rates, breast and ovarian cancer incidence, and mortality rates, and costs in terms of 2009 dollars, on published studies and data from the Surveillance, Epidemiology, and End Results (SEER) Program and the Centers for Medicare and Medicaid Services. We used preference ratings obtained from mutation carriers and controls to adjust survival for quality of life (QALYs). Results: For BRCA1 mutation carriers, prophylactic oophorectomy at $1,741 per QALY, was more cost effective than both surgeries and dominated all other interventions. For BRCA2 carriers, prophylactic oophorectomy, at $4,587 per QALY, was more cost effective than both surgeries. Without quality adjustment, both mastectomy and BSO surgeries dominated all other interventions. In all simulations, preventive surgeries or chemoprevention dominated or were more cost effective than screening because screening modalities were costly. Conclusion: Our analysis suggested that among BRCA1/2 mutation carriers, prophylactic surgery would dominate or be cost effective compared to chemoprevention and screening. Annual screening with MRI and mammography was the most effective strategy because it was associated with the longest quality-adjusted survival, but it was also very expensive.
Keywords: Comparative effectiveness, Cost-effectiveness, Mastectomy, Oophorectomy or both, Tamoxifen, Screening with MRI and mammography, BRCA1/BRCA2
Introduction
Among women with BRCA1 or BRCA2 genetic mutations, contrast-enhanced magnetic resonance imaging (MRI) combined with mammography has been recommended by the American Cancer Society and other authoritative groups for breast screening [1]. Many women who test positive for these mutations now choose to be followed with annual MRI combined with mammography rather than using chemopreventive agents or undergoing prophylactic mastectomy [2–6]. Although randomized trials have not been conducted, observational studies have found that prophylactic mastectomy and/or prophylactic bilateral salpingoophorectomy (BSO) can delay or prevent cancers of the breast and ovary among mutation carriers [7–10]. Mammography alone has not been found to be reliable enough for screening BRCA mutation carriers, in part, because they are at risk for breast cancer at much younger ages than non-carriers, and mammography does not accurately detect cancer in the dense breasts of young women. Recent observational studies have found that screening by MRI with mammography was effective in detecting earlystage breast cancers among BRCA1/2 mutation carriers [11–15]. Currently, mutation carriers who have not been diagnosed with cancer may choose among or combine several preventive strategies: primary prevention with chemopreventive agents (e.g., tamoxifen), prophylactic mastectomy or prophylactic BSO; and secondary prevention with mammography and MRI [2, 16]. Comparative effectiveness analysis is increasingly being used to determine the relative merits of therapeutic interventions in specific patient populations. In a previous analysis, we showed that among mutation carriers, BSO with or without mastectomy was more cost effective than surveillance with annual mammograms [17–19]. Other analyses compared MRI with mammography to mammography alone and found that, although expensive, limited use of MRI might be more cost effective than mammography [20–22]. Using recent cancer risk data on women with BRCA1 and BRCA2 mutations, we have now developed new models to estimate the comparative effectiveness, including quality-adjusted and unadjusted cost effectiveness, of the primary and secondary preventive interventions available to mutation carriers. For quality adjustment, we have used new preference ratings obtained from both women without known high risk and a Canadian cohort of BRCA1 or BRCA2 mutation carriers [23, 24].
Methods
We developed a Markov process [25] and used 25,000 Monte Carlo simulations with TreeAge ProSuite 2009 to estimate the survival, quality-adjusted survival, and costs associated with preventive interventions for BRCA1 and BRCA2 mutation carriers who had no cancer diagnosis at baseline [26]. The interventions (Fig. 1) were prophylactic mastectomy, prophylactic BSO, Prophylactic mastectomy and BSO (both surgeries), tamoxifen, mammography, mammography plus MRI (MRI), and prophylactic BSO plus MRI. (We assumed that women who had MRI would also have mammography because screening with both modalities is now the standard of care for women aged 30+ years, who have a BRCA1/2 mutation [2].) In previous studies, we assumed that mammography alone was the standard of care. We chose five health states as outcomes: good health, breast cancer, ovarian cancer, both breast and ovarian cancer, their complications, and death. We used 25,000 simulations for the base case of this study and followed individuals for new primary breast and ovarian cancers from age 30 (base case) to 65 for survival. We assumed that women who had prophylactic surgery did so at age 35 and that all women started screening with MRI or mammogram at age 30 [27]. For each year of each strategy, we calculated the age-dependent probabilities of developing breast cancer, developing ovarian cancer, dying from breast or ovarian cancer, dying from any cause, or remaining well. We followed all women up to age 100 or death.
Fig. 1.
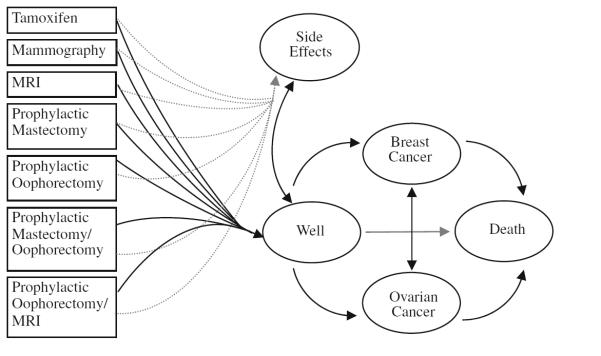
Markov model of BRCA1/2 health states
Health parameters
We included in our model published estimates of the cumulative incidence of breast cancer and ovarian cancer among BRCA1/2-positive women by decade [28]. We converted these 10-year risks to annual conditional probabilities of cancer, assuming constant instantaneous increases in incidence rates per year (Table 1) [19].
Table 1.
Incidence, preventive strategy risk reduction, and mortality assumptions used in the Markov model
| Variable | Value |
|---|---|
| Health states per 100 persons per year ± SE, n | |
| Breast cancer [19, 28] | |
| BRCA 1 mutation carrier | 3.32 ± 0.63 |
| BRCA 2 mutation carrier | 3.79 ± 1.07 |
| BRCA 1 and BRCA 2 | 3.43 ± 0.556 |
| Ovarian cancer [19, 28] | |
| BRCA 1 mutation carrier | 1.55 ± 0.304 |
| BRCA 2 mutation carrier | 0.523 ± 0.031 |
| BRCA 1 and BRCA 2 | 1.12 ± 0.285 |
| Endometrial cancer due to tamoxifen [33] | 0.401 ± 0.019 |
| Pulmonary embolism due to tamoxifen [33] | 0.320 ± 0.180 |
| Cataracts due to tamoxifen [33] | 0.110 ± 0.050 |
| Preventive strategies ± SE, % | |
| Breast cancer risk reduction due to | |
| Prophylactic bilateral mastectomy [19, 51] | 90 ± 5 |
| Mastectomy and oophorectomy [9, 51] | 95 ± 5 |
| Tamoxifen [19, 33] | 49 ± 7 |
| Oophorectomy before age 50 years [8, 9, 37, 52] |
47 ± 1 |
| Ovarian cancer risk reduction due to | |
| Oophorectomy BRCA1 [6, 8, 36, 37] | 96 ± 3 |
| Oophorectomy BRCA2 [6, 8, 36] | 96 ± 3* |
| Oral contraceptives [47] | 54 ± 11 |
| Estrogen receptor+ by age and mutation % [35] | |
| BRCA1 | |
| 30–49 | 18% ± 2 |
| 50–69 | 22% ± 2 |
| ≥70 | 24% ± 2 |
| BRCA2 | |
| 30–49 | 62% ± 2 |
| 50–69 | 75% ± 2 |
| ≥70 | 83% ± 2 |
| Mortality | |
| Breast cancer | 1 – SEER survival rates ≤0–16 years after diagnosis; U.S. population mortality rates thereafter [29] |
| Ovarian cancer | 1 – SEER survival rates ≤0–10 years after diagnosis; U.S. population mortality rates thereafter [29] |
| Endometrial cancer | 1 – SEER survival rates ≤5 years after diagnosis; U.S. population rates >5 years, includes 15% of mixed Mullarian tumors 0–5 years after diagnosis [31]; U.S. population rates [29, 33] |
| Pulmonary embolism | 3% in first year; U.S. population rates after 1 year [29, 33] |
| Cataracts | U.S. population rates [29, 33] |
No ovarian cancer in one study of BRCA2+ women, but not statistically significant. Assumed 96 ± 0.03 [8]
We assumed that among BRCA1/2 mutation carriers, those with breast cancer had the same conditional probability of developing ovarian cancer as those who were well. Using the U.S. National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program data for the period 1973–2004, we also computed estimates of dying with and without cancer [29]. Assuming that BRCA1/2-positive women who developed cancer would have the same conditional probability of death as women with cancer in the general population, we used SEER data to calculate the probabilities of dying [29]. We did not adjust our ovarian cancer survival estimates for screening because heightened surveillance does not appear to alter the prognosis of this cancer [30–32].
For women diagnosed with breast cancer, who were not being screened by MRI, we based our stage distribution assumptions on the cases among participants in the Breast Cancer Prevention Trial (BCPT). Of the BCPT participants in the control arm who developed breast cancer, 70% had localized (node-negative) and 30% regional (node-positive) disease [33].
We assumed that patients who had annual MRI screening would have the same stage distribution as the cases diagnosed in a cohort of 236 BRCA1 and BRCA2 mutation carriers, aged 25–65 years, who underwent annual MRI imaging, ultrasound, mammography, and clinical breast examinations from November 3, 1997 to March 31, 2003 [2, 3, 14, 34 We applied to our model the distributions of pathological TNM stages, tumor size, lymph node status, grade, and hormone receptor status observed in their cancers [14, 35]. For both groups, we computed mortality risk based on SEER data for those distributions [29].
We updated our estimates of the health effects of preventive strategies to reflect the findings of studies published since our previous report. These studies suggest that prophylactic BSO may reduce the risk of ovarian cancer by 96% for BRCA1 and BRCA2 mutation carriers, respectively, and may reduce the risk of breast cancer among premenopausal women by 53% for both [8, 36, 37]. Other studies suggest that prophylactic bilateral mastectomy may reduce the risk of breast cancer by 90% [8]. Table 1 describes our risk assumptions. For our base case, the risk reductions associated with these strategies were assumed to last indefinitely.
We assumed that after prophylactic oophorectomy, most women would take hormone replacement therapy (HRT) until age 50, and that HRT would not affect their risks of breast cancer, cardiovascular disease, or osteoporosis [38].
Cost parameters
We obtained age-stratified data on screening and cancer care costs from the literature and expressed the costs in terms of 2009 US dollars (Table 2). Drug cost data were obtained from the Drug Topics Redbook: Pharmacy’s Fundamental Reference [39]. Patient care costs associated with cancer were obtained from the literature and are given in terms of 2009 dollars.
Table 2.
| Variable (reference) | Total costs (direct + indirect), US$ |
|---|---|
| First year after diagnosis [53–59] | 22,375b (DCIS) 74,149b (breast cancer) 107,619b (ovarian cancer) |
| Subsequent yearly costs [54, 56, 58–60] | 6,506c (breast cancer) 11,831b (ovarian cancer) |
| Terminal care costs, last year of life [54, 58–61] | 54,991 (breast cancer) 75,188 (ovarian cancer) |
| Surveillance (per NCCN guidelines) [61] | 4,476 (without breast or ovarian cancer) |
| Terminal care costs, last year of life [59, 60, 62] | 36,199 (without breast or ovarian cancer) |
| Other medical costsb | |
| Endometrial cancerb [19] | 6,211 |
| Pulmonary embolib [19, 39] | 5,173 |
| Cataract surgeryb [19] | 3,987 |
| Genetic testing and counseling [63] | 3,175 |
| Preventive strategies | |
| Tamoxifen cost per year for premenopausal women (for 5 years) [39] | 727 |
| Letrozole cost per year for postmenopausal women (for 5 years) [39, 64] | 3,516 |
| Prophylactic mastectomy [5, 6] | 10,591 |
| Prophylactic salpingo-oophorectomy [5, 6] | 6,373 |
| Both prophylactic mastectomy and salpingo-oophorectomy | 16,964 |
| Prophylactic salpingo-oophorectomy and tamoxifen <age 50 (includes cost per year for 5 years of tamoxifen) [39] |
10,008 |
| Screening strategies including work and 2009 dollars [20, 27, 54, 65] | |
| Bilateral screening mammogram | |
| Initial screening mammogram | 129 |
| Follow-up mammogram | 120 |
| Ultrasound | 112 |
| Mammographic-guided surgical biopsy | 1,667 |
| Ultrasound-guided core needle biopsy | 716 |
| Stereotactic biopsy | 997 |
| Ultrasound-guided fine needle biopsy | 581 |
| Average total diagnostic cost following mammographic screening | 31 |
| Bilateral screening MRI | |
| Initial screening MRI | 1,219 |
| Short interval follow-up MRI | 940 |
| MRI-guided surgical biopsy | 2,131 |
| MRI-guided core needle biopsy | 1,199 |
| Average total diagnostic cost following initial MRI | 373 |
| Average total diagnostic cost following subsequent MRI | 209 |
The discount rate (range) was 3% (0–5%). DRG diagnosis-related group, NCCN National Comprehensive Cancer Network, V/Q ventilation-perfusion
Adjusted by the Medical Consumer Price Index into 2009 US$ [65]
Yearly costs for patients with breast cancer apply to years 2–16 after diagnosis. Cost for years >16 are the same as those for healthy women
Quality-of-life adjustment
We adjusted our survival estimates for quality of life based on preference ratings of cancer- and preventive treatment-related states obtained from both mutation carriers in the previously described MRI cohort and women without known high risk (Table 3) [14, 35, 40]. We interpreted the preferences of the latter group as representing a societal standard. The preference ratings were derived from responses to a time trade-off questionnaire, which presented vignettes or scenarios involving various cancer-related states and asked respondents how much of their life expectancy they would trade to avoid those states. The states were breast cancer, ovarian cancer, bilateral mastectomy, BSO, both surgeries, use of a chemopreventive medication (tamoxifen), mammography, and MRI. The cancer state vignettes quantified the risk of recurrence and the effect of the cancer on life expectancy. Each vignette was based on literature review, patient interviews, and professional oncological experience. The preference rating was calculated as total life expectancy minus traded time, divided by total life expectancy.
Table 3.
Preference ratings used for quality adjustment
| Health states (reference) | Mean (SD) Controls |
Preference ratings Mutation carriers |
|---|---|---|
| Perfect healtha | 1.00 | 1.00 |
| Cancer states among high-risk womena | ||
| Breast cancer | 0.84 (0.18) | 0.87 (0.20) |
| Ovarian cancer | 0.83 (0.17) | 0.84 (0.23) |
| Preventive measuresa | ||
| Tamoxifen | 0.90 (0.16) | 0.95 (0.14) |
| Prophylactic mastectomy | 0.88 (0.17) | 0.88 (0.22) |
| Prophylactic bilateral salpingo-oophorectomy | 0.90 (0.14) | 0.95 (0.10) |
| Both prophylactic surgeries | 0.79 (0.21) | 0.84 (0.23) |
| Mammogram | 0.97 (0.11) | 1.00 (0.004) |
| MRI | 0.96 (0.13) | 1.00 (0.005) |
| MRI and BSO | 0.86 (0.18) | 0.95 (0.01) |
| Other health states associated with tamoxifenb | ||
| Endometrial cancer [19] | 0.68 | 0.68 |
| Pulmonary Emboli [19] | 0.50 | 0.50 |
| Cataract surgery [19] | 0.68 | 0.68 |
| Well with positive BRCA1 or BRCA2 test resulta [40] | 0.87 (0.16) | 0.92 (0.15) |
| Death | 0.00 | 0.00 |
Comparative effectiveness analysis
We computed the mean cost (SD) of each intervention for a BRCA1 or BRCA2 mutation carrier to those who chose it. We then listed the interventions in the order of costs from the least to the most expensive. We computed the incremental cost of each intervention over that of the next most expensive one. We computed the mean survival (SD) in life years (LYs) or quality-adjusted life years (QALYs) associated with each intervention, and their incremental LYs or QALYs (positive or negative) above that associated with the least expensive intervention. Interventions that were more expensive and less effective (in LYs or QALYs) were designated as dominated. Extended dominance represents the percentage of the population that receives the less effective treatment and is considered dominated (Appendix Figure 4).
Results
Table 4 presents the results of the comparative effectiveness analyses, which we conducted separately for BRCA1 and BRCA2 mutation carriers. Based on the preferences of women without known high risk for breast or ovarian cancer, the optimal strategy for BRCA1 mutation carriers was BSO, with an incremental cost effectiveness ratio (ICER) of $1,741 per QALY compared to both surgeries; for BRCA2 mutation carriers, BSO had an ICER of $4,587(Table 4). With quality adjustment based on mutation carriers’ preferences, the optimal strategy for BRCA1 mutation carriers was still BSO, with an ICER of $1,677 per QALY. For BRCA2 mutation carriers, prophylactic oophorectomy had extended dominance over bilateral mastectomy with an ICER of $4,535 per QALY (Table 4). MRI plus BSO was associated with the most QALYs of any strategy for BRCA1 carriers, but at a cost of $170,899 and an unacceptable ICER of $736,788 per QALY compared to prophylactic oophorectomy alone.
Table 4.
Estimated cost effectiveness of screening and primary preventive interventions for BRCA1 and BRCA2 mutation carriers
| Intervention | Mean (SD) cost in per subject |
Incremental cost |
Mean (SD) QALYs |
Incremental QALYs |
Incremental cost/ incremental QALYs |
|---|---|---|---|---|---|
| With quality-adjusted survival in life years (QALYs) based on preference ratings of women without known high risk | |||||
| BRCA1 | |||||
| Both prophylactic surgeries | $150,986 ($3,152) | – | 16.66 (2.80) | – | – |
| Prophylactic oophorectomy | $153,396 ($2,945) | $2,410 | 18.04 (1.43) | 1.38 | $1,741 |
| Prophylactic mastectomy | $167,607 ($3,709) | $14,211 | 17.52 (1.65) | −0.52 | Dominated |
| Prophylactic oophorectomy and MRI | $170,893 ($3,393) | $17,497 | 17.63 (1.90) | −0.41 | Dominated |
| Tamoxifen | $172,353 ($3,887) | $18,957 | 17.43 (1.38) | −0.61 | Dominated |
| Mammography | $179,639 ($4,098) | $26,243 | 18.08 (0.96) | −0.40 | $681,333 |
| Mammography and MRI | $192,429 ($4,169) | $12,790 | 18.08 (1.01) | −0.01 | Dominated |
| BRCA2 | |||||
| Both prophylactic surgeries | $140,684 ($3,387) | – | 16.82 (1.60) | – | – |
| Prophylactic mastectomy | $146,505 ($3,378) | $5,821 | 17.97 (2.89) | 1.15 | Extended dominancea |
| Prophylactic oophorectomy | $147,106 ($3,586) | $6,422 | 18.22 (1.79) | 1.40 | $4,587 |
| Tamoxifen | $154,725 ($3,197) | $7,618 | 17.81 (1.47) | −0.41 | Dominated |
| Prophylactic oophorectomy and MRI | $164,039 ($3,465) | $16,932 | 17.84 (1.97) | −0.39 | Dominated |
| Mammography | $165,843 ($4,123) | $18,736 | 18.44 (1.07) | 0.21 | $88,104 |
| Mammography and MRI | $177,918 ($4,163) | $12,075 | 18.49 (1.13) | 0.49 | $247,645 |
| With quality-adjusted survival in life years (QALYs) based on preference ratings of BRCA1/2 mutation carriers | |||||
| BRCA1 | |||||
| Both prophylactic surgeries | $151,048 ($3,152) | – | 17.49 (2.83) | – | – |
| Prophylactic oophorectomy | $153,395 ($2,945) | $2,347 | 18.89 (2.83) | 1.40 | $1,677 |
| Prophylactic mastectomy | $167,559 ($3,709) | $14,163 | 17.64 (1.65) | −1.25 | Dominated |
| Prophylactic oophorectomy and MRI | $170,899 ($3,393) | $17,504 | 18.92 (1.90) | 0.02 | $736,788 |
| Tamoxifen | $172,321 ($3,887) | $1,422 | 18.22 (1,38) | −0.70 | Dominated |
| Mammography | $179,617 ($4,098) | $8,718 | 18.55 (0.96) | −0.37 | Dominated |
| Mammography and MRI | $192,418 ($4,169) | $21,519 | 18.66 (1.01) | −0.26 | Dominated |
| BRCA2 | |||||
| Both prophylactic surgeries | $140,674 ($3,387) | – | 17.68 (1.60) | – | – |
| Prophylactic mastectomy | $146,440 ($3,378) | $5,766 | 18.10 (2.89) | 0.41 | Extended dominancea |
| Prophylactic oophorectomy | $147,069 ($3,586) | $6,395 | 19.09 (1.79) | 1.41 | $4,535 |
| Tamoxifen | $154,681 ($3,197) | $7,612 | 18.70 (1.47) | −0.43 | Dominated |
| Prophylactic oophorectomy and MRI | $164,045 ($3,465) | $16,976 | 19.16 (1.97) | 0.072 | $236,867 |
| Mammography | $165,760 ($4,123) | $1,714 | 18.94 (1.07) | −0.23 | Dominated |
| Mammography and MRI | $177,934 ($4,163) | $13,888 | 19.12 (1.13) | −0.05 | Dominated |
| With survival in life years (LYs) only (no quality adjustment) | |||||
| BRCA1 | |||||
| Both prophylactic surgeries | $151,023 ($2,959) | – | 20.65 (0.53) | – | – |
| Prophylactic oophorectomy | $153,387 ($3,145) | $2,365 | 20.38 (0.51) | −0.27 | Dominated |
| Prophylactic mastectomy | $167,553 ($3,730) | $16,531 | 20.11 (0.50) | −0.54 | Dominated |
| Prophylactic oophorectomy and MRI | $170,881 ($3,380) | $19,859 | 20.48 (0.51) | −0.17 | Dominated |
| Tamoxifen | $172,327 ($3,886) | $21,304 | 19.86 (0.48) | −0.79 | Dominated |
| Mammography | $179,628 ($4,065) | $28,605 | 19.70 (0.47) | −0.95 | Dominated |
| Mammography and MRI | $192,402 ($4,184) | $41,380 | 19.83 (0.48) | −0.82 | Dominated |
| BRCA2 | |||||
| Both prophylactic surgeries | $140,688 ($3,394) | – | 20.87 (0.52) | – | – |
| Prophylactic mastectomy | $146,528 ($3,882) | $5,840 | 20.57 (0.53) | −0.30 | Dominated |
| Prophylactic oophorectomy | $147,082 ($3,810) | $6,394 | 20.56 (0.52) | −0.31 | Dominated |
| Tamoxifen | $154,738 ($3,979) | $14,049 | 20.24 (0.50) | −0.63 | Dominated |
| Prophylactic oophorectomy and MRI | $163,997 ($3,461) | $23,309 | 20.70 (0.50) | −0.17 | Dominated |
| Mammography | $165,803 ($4,175) | $25,115 | 20.03 (0.49) | −0.84 | Dominated |
| MRI and mammography | $177,929 ($5,004) | $37,240 | 20.23 (0.50) | −0.64 | Dominated |
Without quality adjustment, using LYs saved, the optimal strategy adopted for both BRCA1 and BRCA2 was prophylactic surgery, as it was the most effective and the least expensive (Table 4).
Mammogram versus MRI
In the United States, MRI costs nearly ten times as much as mammography alone (Table 2). If the cost of MRI among BRCA1 carriers were reduced by 30% and that among BRCA2 carriers by 10%, then MRI would be cost effective compared to mammography (Appendix Table 1). At a 70% cost reduction, MRI would dominate (i.e., would be less costly and would provide better survival than) mammography alone.
If penetrance approximated (Appendix: Table 2) the cancer incidence rates of women in the general population, mammograms would dominate MRI. If penetrance increased, then prophylactic surgery would dominate, but the benefit would differ according to BRCA1 or BRCA2 status.
Costs and effectiveness of our base case was 3%, but would decrease progressively in our sensitivity analysis (Appendix: Table 3) with an increase of 0–5% in the discount rates.
At all ages (Appendix: Table 4) among BRCA1 or BRCA2 carriers, both prophylactic surgeries would dominate other strategies in life years saved, but the benefit would decrease with quality adjustment.
Variability in both costs and effectiveness outcomes is demonstrated (Appendix: Figures 2 and 3) using 25,000 second-order probabilistic simulations of the rates. Annual MRI and mammography screening is more expensive and has greater quality-adjusted effectiveness compared to the five prevention strategies.
Discussion
To our knowledge, this is the first study conducted to analyze the comparative effectiveness of screening and primary prevention strategies among women who have tested positive for BRCA1/2 mutations. For this analysis, we carefully followed guidelines on as to how to do the analysis as outlined by the American College of Physicians and Institute of Medicine [24]. The Congressional Budget Office may have had in mind such analyses of quality and cost [24, 41] in preparing its recent report submitted to health policy makers about the costs of suboptimal health care [42, 43]. Mutation carriers gave high preference ratings to MRI, but although it leads to the diagnosis of smaller cancers [2, 3], no randomized trial has shown that it prolongs life [22, 44]. In short-term studies among younger women, it appears cost effective compared to mammography alone [20].
Before the widespread use of MRI, we conducted a cost-effectiveness analysis comparing genetic testing plus preventive surgical interventions with surveillance alone for BRCA1/2 mutation carriers [19]. We showed that from a societal perspective, with unadjusted survival (LYs saved) as the outcome, BRCA testing followed by surgical intervention for those who tested positive was the most effective option.
In this study, we compared preventive surgery, chemoprevention, MRI, and mammography. Our outcomes were costs, life-years (survival), and quality-adjusted life-years. Quality adjustment takes into account the morbidity, emotional distress, and inconvenience that the preventive interventions entail [45, 46], which vary among individuals depending on their personalities and circumstances. As Table 3 indicates, mutation carriers and controls (women without known high risk) differed only slightly in their preference ratings [40]. Overall, study participants’ preference ratings of each health state varied considerably, and our Monte Carlo simulations reflected this variation (Appendix: Figures 2 and 3). Quality adjustment had a tremendous impact on our cost-effectiveness estimates. The strategy of prophylactic mastectomy and oophorectomy had the lowest overall cost. It dominated all other strategies and had the longest survival in LYs, but the lowest preference ratings. These findings are similar to those reported in a recent study of an international group of 2,677 BRCA mutation carriers [16].
Conversely, MRI ? mammogram was the most costly intervention for both BRCA1 and BRCA2 mutations carriers, but had the highest QALYs from a societal perspective (Table 4) for both BRCA1 and BRCA2 carriers. In sensitivity analysis, if the cost of MRI was reduced by 75% to $305, then annual imaging was cost effective compared to mammogram alone. Similarly, Moore et al. recently reported an ICER of less than $50,000 per QALY for MRI at a cost <$315, compared to mammogram alone among women followed for over 25 years [22]. Without this cost reduction, the ICER of MRI was $179,599 compared to mammography alone, similar to our estimates of a follow up over a a 35-year period.
In our simulated cohort, using the preferences of BRCA1 and BRCA2 mutation carriers (Table 4), the most favorable ICERs of this study were prophylactic oophorectomy for BRCA1 at $1,677 per QALY and BRCA2, at $4,535 per QALY. Despite their high preference ratings, the imaging modalities were dominated by preventive surgeries because MRI and mammography are recurring, expensive annual events, which add up to the expense of diagnosis and treatment [20] compared to prevention. Both modalities, especially MRI, result in many false positives, which can lead to psychological stress and unnecessary biopsies [3]. Over time, however, the readings may become more accurate and effective in differentiating benign tissue from cancer [2, 3].
The analyses performed previously by us have shown that outcomes vary depending on the ages of those being tested for BRCA1/2, the penetrance and prevalence of the mutations, the efficacy of preventive strategies, the preference ratings applied to those strategies, the morbidity and mortality of the disease itself, and the accuracy of screening (positive and negative predictive value) [2, 17].
In a previous model of ours, age remained an important predictor of comparative effectiveness from a health policy perspective. Many women are reluctant to have prophylactic oophorectomy before age 35 or until they have had a family. However, the previous research performed by us indicates that the cost effectiveness of testing rises, and therefore worsens, rapidly as the age at screening and prevention increases [19]. Women can postpone decisions regarding oophorectomy using oral contraceptives for a number of years and postpone mastectomy using MRI to identify early-stage cancers [47, 48]. The analysis in this study indicates that in a high-risk population, screening with MRI is effective (Table 4), although expensive. Many women with BRCA2 mutations attending high-risk clinics are offered tamoxifen, which, especially if used after oophorectomy, may reduce breast cancer risk [10], but some women and physicians are reluctant to use tamoxifen because of its potential side effects and the lack of evidence that it benefits mutation carriers specifically [49]. Trials of chemopreventive strategies including tamoxifen, raloxifene, and aromatase inhibitors are needed among populations at increased risk, such as Ashkenazi Jewish women with family histories of cancer.
A possible limitation of this study is the lack of data supporting our assumptions about stage distribution, given the screening by MRI and mammography versus mammography alone. We assumed that the stage distribution of breast cancer among women who received MRI would be the same as that of the cases in a cohort who received yearly MRI and mammography [2, 3, 14] and that the stage distribution among women who had mammography alone would be the same as that among high-risk women in the control arm of the NSABP BCPT tamoxifen trial [33], who had yearly mammograms. These assumptions may be biased because the women in both groups were trial participants and may, therefore, have had more careful surveillance than might be expected outside a research setting, but in our model, the difference had little effect on survival.
Both payers and policy makers should be aware of the potential benefits of MRI imaging and make efforts to bring the costs of screening down for all the high-risk women who wish to forego preventive surgery, at least for a set amount of time. In Canada, MRI costs 20% less than in the United States, and in England the cost may be even lower. Many women may wish to combine MRI with prophylactic oophorectomy, as we have done in our modeling. It may also be possible to conduct randomized trials to test the effects of tamoxifen, raloxifene, and aromatase inhibitors, with and without prophylactic oophorectomy.
The strategies that we analyzed varied little in overall effectiveness (Appendix: Figures 2 and 3). The differences in cost effectiveness were driven by differences in cost, especially the high cost of annual MRI. In our models, MRI was the most effective strategy because of its high preference rating and because its high sensitivity was associated with long survival. However, it was by far the most expensive of the strategies because it involves a costly and recurrent procedure and because it leads to many negative biopsies and possible overdiagnosis. Weinstein and Skinner [50] recently described the complexity of controlling costs given such constraints as geography, the structure of health care, and the value of particular treatments to the patient.
For known mutation carriers, preventive options are now a reality. Given that randomized trials comparing those options are unlikely to be conducted in the foreseeable future, we hope that our model results will provide policy makers and health care providers with some interim guidance. However, we should at least conduct observational studies to track utilization and to improve assessment of the available preventive options.
Average-risk women, especially those who are premenopausal, also need better preventive measures. We now know that breast and ovarian cancer can be prevented. We need to build on this knowledge to develop more evidencebased, acceptable, and affordable strategies to improve cancer outcomes.
Supplementary Material
Acknowledgment
This study was supported by Grant #: RSGHP-03-166-01-PBP Research Scholar Grant from the American Cancer Society, Atlanta, GA. (SpinOdyssey) Westport, CT and the Avon Family Foundation. Dr. Hershman is the recipient of a grant from the National Cancer Institute (NCI R01CA134964).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-010-1043-4) contains supplementary material, which is available to authorized users.
Contributor Information
Victor R. Grann, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA; Department of Health Policy and Management, Joseph L. Mailman School of Public Health, Columbia University, New York, NY, USA.
Priya R. Patel, Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Judith S. Jacobson, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA Department of Epidemiology, Joseph L. Mailman School of Public Health, Columbia University, Room 734, 722 West 168th Street, New York, NY 10032, USA.
Ellen Warner, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
Daniel F. Heitjan, Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Maxine Ashby-Thompson, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA.
Dawn L. Hershman, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA; Department of Epidemiology, Joseph L. Mailman School of Public Health, Columbia University, Room 734, 722 West 168th Street, New York, NY 10032, USA.
Alfred I. Neugut, Herbert Irving Comprehensive Cancer Center, Columbia University, New York, NY, USA Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA; Department of Epidemiology, Joseph L. Mailman School of Public Health, Columbia University, Room 734, 722 West 168th Street, New York, NY 10032, USA.
References
- 1.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, Morris E, Pisano E, Schnall M, Sener S, Smith RA, Warner E, Yaffe M, Andrews KS, Russell CA. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 2.Warner E. The role of magnetic resonance imaging in screening women at high risk of breast cancer. Top Magn Reson Imaging. 2008;19(3):163–169. doi: 10.1097/RMR.0b013e31818bc994. doi:10.1097/RMR.0b013e31818bc9940 0002142-200806000-00003[pii] [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med. 2008;148(9):671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann LC, Sellers TA, Schaid DJ, Frank TS, Soderberg CL, Sitta DL, Frost MH, Grant CS, Donohue JH, Woods JE, Mc-Donnell SK, Vockley CW, Deffenbaugh A, Couch FJ, Jenkins RB. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93(21):1633–1637. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann LC, Degnim A, Schaid DJ. Prophylactic mastectomy for BRCA1/2 carriers: progress and more questions. J Clin Oncol. 2004;22(6):981–983. doi: 10.1200/JCO.2004.01.925. doi:10.1200/JCO.2004.01.925JCO. 2004.01.925[pii] [DOI] [PubMed] [Google Scholar]
- 6.Rebbeck TR, Domchek SM. Variation in breast cancer risk in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2008;10(4):108. doi: 10.1186/bcr2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kauff ND, Barakat RR. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J Clin Oncol. 2007;25(20):2921–2927. doi: 10.1200/JCO.2007.11.3449. [DOI] [PubMed] [Google Scholar]
- 8.Kauff ND, Domchek SM, Friebel TM, Robson ME, Lee J, Garber JE, Isaacs C, Evans DG, Lynch H, Eeles RA, Neuhausen SL, Daly MB, Matloff E, Blum JL, Sabbatini P, Barakat RR, Hudis C, Norton L, Offit K, Rebbeck TR. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: a multicenter, prospective study. J Clin Oncol. 2008;26(8):1331–1337. doi: 10.1200/JCO.2007.13.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rebbeck TR, Friebel T, Lynch HT, Neuhausen SL, Van’t Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff E, Daly MB, Olopade OI, Weber BL. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: the prose study group. J Clin Oncol. 2004;22(6):1055–1062. doi: 10.1200/JCO.2004.04.188. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, Olopade OI, Eisen A, Weber B, McLennan J, Sun P, Foulkes WD, Narod SA. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004;22(12):2328–2335. doi: 10.1200/JCO.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 11.Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351(5):427–437. doi: 10.1056/NEJMoa031759. [DOI] [PubMed] [Google Scholar]
- 12.Kriege M, Brekelmans CT, Peterse H, Obdeijn IM, Boetes C, Zonderland HM, Muller SH, Kok T, Manoliu RA, Besnard AP, Tilanus-Linthorst MM, Seynaeve C, Bartels CC, Meijer S, Oosterwijk JC, Hoogerbrugge N, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG. Tumor characteristics and detection method in the mrisc screening program for the early detection of hereditary breast cancer. Breast Cancer Res Treat. 2007;102(3):357–363. doi: 10.1007/s10549-006-9341-6. doi:10.1007/s10549-006-9341-6. [DOI] [PubMed] [Google Scholar]
- 13.Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, Kuhn W, Schild HH. Mammography, breast ultrasound, and magnetic resonance imagingfor surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476. doi: 10.1200/JCO.2004.00.4960. [DOI] [PubMed] [Google Scholar]
- 14.Warner E, Plewes DB, Hill KA, Causer PA, Zubovits JT, Jong RA, Cutrara MR, DeBoer G, Yaffe MJ, Messner SJ, Meschino WS, Piron CA, Narod SA. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–1325. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 15.Sardanelli F, Podo F, D’Agnolo G, Verdecchia A, Santaquilani M, Musumeci R, Trecate G, Manoukian S, Morassut S, de Giacomi C, Federico M, Cortesi L, Corcione S, Cirillo S, Marra V, Cilotti A, Di Maggio C, Fausto A, Preda L, Zuiani C, Contegiacomo A, Orlacchio A, Calabrese M, Bonomo L, Di Cesare E, Tonutti M, Panizza P, Del Maschio A. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology. 2007;242(3):698–715. doi: 10.1148/radiol.2423051965. [DOI] [PubMed] [Google Scholar]
- 16.Metcalfe KA, Birenbaum-Carmeli D, Lubinski J, Gronwald J, Lynch H, Moller P, Ghadirian P, Foulkes WD, Klijn J, Friedman E, Kim-Sing C, Ainsworth P, Rosen B, Domchek S, Wagner T, Tung N, Manoukian S, Couch F, Sun P, Narod SA. International variation in rates of uptake of preventive options in BRCA1 and BRCA2 mutation carriers. Int J Cancer. 2008;122(9):2017–2022. doi: 10.1002/ijc.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grann VR, Jacobson JS, Thomason D, Hershman DL, Heitjan DF, Neugut AI. Effect of prevention strategies on survival and quality-adjusted survival of women with BRCA1/2 mutations: an updated decision analysis. J Clin Oncol. 2002;20(10):2520–2529. doi: 10.1200/JCO.2002.10.101. [DOI] [PubMed] [Google Scholar]
- 18.Grann VR, Panageas KS, Whang W, Antman KH, Neugut AI. Decision analysis of prophylactic mastectomy and oophorectomy in BRCA1-positive or BRCA2-positive patients. J Clin Oncol. 1998;16(3):979–985. doi: 10.1200/JCO.1998.16.3.979. [DOI] [PubMed] [Google Scholar]
- 19.Anderson K, Jacobson JS, Heitjan DF, Zivin JG, Hershman DL, Neugut AI, Grann VR. Cost-effectiveness of preventive strategies for women with a BRCA1 or a BRCA2 mutation. Ann Intern Med. 2006;144(6):397–406. doi: 10.7326/0003-4819-144-6-200603210-00006. [DOI] [PubMed] [Google Scholar]
- 20.Plevritis SK, Kurian AW, Sigal BM, Daniel BL, Ikeda DM, Stockdale FE, Garber AM. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006;295(20):2374–2384. doi: 10.1001/jama.295.20.2374. [DOI] [PubMed] [Google Scholar]
- 21.Griebsch I, Brown J, Boggis C, Dixon A, Dixon M, Easton D, Eeles R, Evans DG, Gilbert FJ, Hawnaur J, Kessar P, Lakhani SR, Moss SM, Nerurkar A, Padhani AR, Pointon LJ, Potterton J, Thompson D, Turnbull LW, Walker LG, Warren R, Leach MO. Cost-effectiveness of screening with contrast enhanced magnetic resonance imaging vs x-ray mammography of women at a high familial risk of breast cancer. Br J Cancer. 2006;95(7):801–810. doi: 10.1038/sj.bjc.6603356. doi:6603356[pii]10.1038/sj.bjc.6603356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore SG, Shenoy PJ, Fanucchi L, Tumeh JW, Flowers CR. Cost-effectiveness of MRI compared to mammography for breast cancer screening in a high risk population. BMC Health Serv Res. 2009;9:9. doi: 10.1186/1472-6963-9-9. doi:1472-6963-9-9[pii]10.1186/1472-6963-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sox HC. The evaluation of diagnostic tests: principles, problems, and new developments. Annu Rev Med. 1996;47:463–471. doi: 10.1146/annurev.med.47.1.463. [DOI] [PubMed] [Google Scholar]
- 24.Sox HC, Greenfield S. Comparative effectiveness research: a report from the institute of medicine. Ann Intern Med. 2009;151(3):203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. doi:0000605-200908040-00125[pii] [DOI] [PubMed] [Google Scholar]
- 25.Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–458. doi: 10.1177/0272989X8300300403. [DOI] [PubMed] [Google Scholar]
- 26.TreeAge . Pro 2009 Release 1.02. 1.02 edn TreeAge Software, Inc.; Williamstown: 2009. [Google Scholar]
- 27.Warner E, Plewes D, Hill K, Causer P, Deboer G, Narod S, Cutrara M, Ramsay E. Effect of age and temporal patterns over 5 years in a magnetic resonance imaging (MRI)-based breast surveillance study for BRCA mutation carriers. 2004 ASCO annual meeting; New Orleans. 2004. abstract no. 9500. [Google Scholar]
- 28.King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 29.Surveillance, Epidemiology, and End Results (SEER) Program. ( www.Seer.Cancer.Gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use, Nov 2006 submission (1973–2004 varying), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 30.Swisher E. Prophylactic surgery and other strategies for reducing the risk of familial ovarian cancer. Curr Treat Options Oncol. 2003;4(2):105–110. doi: 10.1007/s11864-003-0011-1. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PE. Nongenetic screening of ovarian malignancies. Obstet Gynecol Clin North Am. 2001;28(4):637–651. doi: 10.1016/s0889-8545(05)70226-6. [DOI] [PubMed] [Google Scholar]
- 32.Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med. 2009;6(7):e1000114. doi: 10.1371/journal.pmed.1000114. doi:10.1371/journal.pmed.1000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 34.Bigenwald RZ, Warner E, Gunasekara A, Hill KA, Causer PA, Messner SJ, Eisen A, Plewes DB, Narod SA, Zhang L, Yaffe MJ. Is mammography adequate for screening women with inherited BRCA mutations and low breast density? Cancer Epidemiol Biomarkers Prev. 2008;17(3):706–711. doi: 10.1158/1055-9965.EPI-07-0509. [DOI] [PubMed] [Google Scholar]
- 35.Chappuis PO, Rosenblatt J, Foulkes WD. The influence of familial and hereditary factors on the prognosis of breast cancer. Ann Oncol. 1999;10(10):1163–1170. doi: 10.1023/a:1008301314812. [DOI] [PubMed] [Google Scholar]
- 36.Rebbeck TR, Lynch HT, Neuhausen SL, Narod SA, Van’t Veer L, Garber JE, Evans G, Isaacs C, Daly MB, Matloff E, Olopade OI, Weber BL. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346(21):1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 37.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101(2):80–87. doi: 10.1093/jnci/djn442. doi:djn442[pii]10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22(6):1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 39.Red Book-Pharmacy’s Fundamental Reference. Thomson PDR; Montvale, NJ: 2008. 07645. [Google Scholar]
- 40.Grann VR, Patel P, Bharthuar A, Jacobson JS, Warner E, Anderson K, Tsai WY, Hill KA, Neugut AI, Hershman DL. Breast cancer-related preferences among women with and without BRCA mutations. Breast Cancer Res Treat. 2010;119(1):177–184. doi: 10.1007/s10549-009-0373-6. doi:10.1007/s10549-009-0373-6. [DOI] [PubMed] [Google Scholar]
- 41.Congressional Budget Office [Accessed Dec 2009];Research on the comparative effectiveness of medical treatments: issues and options for an expanded federal role. 2007 http://www.cbo.gov/ftpdocs/88xx/doc 8891/12-18-ComparativeEffectiveness.pdf.
- 42.Luce BR, Kramer JM, Goodman SN, Connor JT, Tunis S, Whicher D, Schwartz JS. Rethinking randomized clinical trials for comparative effectiveness research: the need for transformational change. Ann Intern Med. 2009;151(3):206–209. doi: 10.7326/0003-4819-151-3-200908040-00126. doi:0000605-200908040-00126[pii] [DOI] [PubMed] [Google Scholar]
- 43.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. doi:NEJMp0904133[pii]10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 44.Wainberg S, Husted J. Utilization of screening and preventive surgery among unaffected carriers of a BRCA1 or BRCA2 gene mutation. Cancer Epidemiol Biomarkers Prev. 2004;13(12):1989–1995. [PubMed] [Google Scholar]
- 45.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 46.Earle CC, Chapman RH, Baker CS, Bell CM, Stone PW, Sandberg EA, Neumann PJ. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18(18):3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 47.Whittemore AS, Balise RR, Pharoah PD, Dicioccio RA, Oakley-Girvan I, Ramus SJ, Daly M, Usinowicz MB, Garlinghouse-Jones K, Ponder BA, Buys S, Senie R, Andrulis I, John E, Hopper JL, Piver MS. Oral contraceptive use and ovarian cancer risk among carriers of BRCA1 or BRCA2 mutations. Br J Cancer. 2004;91(11):1911–1915. doi: 10.1038/sj.bjc.6602239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurian AW, Sigal BM, Plevritis SK. Survival analysis of cancer risk reduction strategies for BRCA1/2 mutation carriers. J Clin Oncol. 2010;28(2):222–231. doi: 10.1200/JCO.2009.22.7991. doi:JCO.2009.22.7991[pii]10.1200/JCO.2009.22.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King MC, Wieand S, Hale K, Lee M, Walsh T, Owens K, Tait J, Ford L, Dunn BK, Costantino J, Wickerham L, Wolmark N, Fisher B. Tamoxifen and breast cancer incidence among women with inherited mutations in BRCA1 and BRCA2: National Surgical Adjuvant Breast and Bowel Project (NSABP-P1) Breast Cancer Prevention Trial. JAMA. 2001;286(18):2251–2256. doi: 10.1001/jama.286.18.2251. [DOI] [PubMed] [Google Scholar]
- 50.Weinstein MC, Skinner JA. Comparative effectiveness and health care spending—implications for reform. N Engl J Med. 2010;362(5):460–465. doi: 10.1056/NEJMsb0911104. doi:NEJMsb0911104[pii]10.1056/NEJMsb0911 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartmann LC, Schaid DJ, Woods JE, Crotty TP, Myers JL, Arnold PG, Petty PM, Sellers TA, Johnson JL, McDonnell SK, Frost MH, Jenkins RB, Grant CS, Michels VV. Efficacy of bilateral prophylactic mastectomy in women with a family history of breast cancer. N Engl J Med. 1999;340(2):77–84. doi: 10.1056/NEJM199901143400201. [DOI] [PubMed] [Google Scholar]
- 52.Rebbeck TR, Levin AM, Eisen A, Snyder C, Watson P, Cannon-Albright L, Isaacs C, Olopade O, Garber JE, Godwin AK, Daly MB, Narod SA, Neuhausen SL, Lynch HT, Weber BL. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91(17):1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 53.Kurian AW, Thompson RN, Gaw AF, Arai S, Ortiz R, Garber AM. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25(6):634–641. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- 54.Yabroff KR, Davis WW, Lamont EB, Fahey A, Topor M, Brown ML, Warren JL. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99(1):14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 55.Barlow WE, Taplin SH, Yoshida CK, Buist DS, Seger D, Brown M. Cost comparison of mastectomy versus breast-conserving therapy for early-stage breast cancer. J Natl Cancer Inst. 2001;93(6):447–455. doi: 10.1093/jnci/93.6.447. [DOI] [PubMed] [Google Scholar]
- 56.Bristow RE, Santillan A, Diaz-Montes TP, Gardner GJ, Giuntoli RL, Meisner BC, II, Frick KD, Armstrong DK. Centralization of care for patients with advanced-stage ovarian cancer: a cost-effectiveness analysis. Cancer. 2007;109(8):1513–1522. doi: 10.1002/cncr.22561. [DOI] [PubMed] [Google Scholar]
- 57.Bristow RE, Santillan A, Salani R, Diaz-Montes TP, Giuntoli RL, II, Meisner BC, Armstrong DK, Frick KD. Intraperitoneal cisplatin and paclitaxel versus intravenous carboplatin and paclitaxel chemotherapy for stage III ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol. 2007;106(3):476–481. doi: 10.1016/j.ygyno.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 58.Rocconi RP, Case AS, Straughn JM, Jr, Estes JM, Partridge EE. Role of chemotherapy for patients with recurrent platinum-resistant advanced epithelial ovarian cancer: a cost-effectiveness analysis. Cancer. 2006;107(3):536–543. doi: 10.1002/cncr.22045. [DOI] [PubMed] [Google Scholar]
- 59.Case AS, Rocconi RP, Partridge EE, Straughn JM., Jr A cost-effectiveness analysis of chemotherapy for patients with recurrent platinum-sensitive epithelial ovarian cancer. Gynecol Oncol. 2007;105(1):223–227. doi: 10.1016/j.ygyno.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 60.Hensley ML, Dowell J, Herndon JE, II, Winer E, Stark N, Weeks JC, Paskett E. Economic outcomes of breast cancer survivorship: CALGB study 79804. Breast Cancer Res Treat. 2005;91(2):153–161. doi: 10.1007/s10549-004-6497-9. [DOI] [PubMed] [Google Scholar]
- 61.Rao S, Kubisiak J, Gilden D. Cost of illness associated with metastatic breast cancer. Breast Cancer Res Treat. 2004;83(1):25–32. doi: 10.1023/B:BREA.0000010689.55559.06. [DOI] [PubMed] [Google Scholar]
- 62.Calfo S, Smith J, Zezza M. Last year of life study. Centers for Medicare and Medicaid Services, Office of the Actuary; [Accessed 22 Aug 2007]. 2005. https://www.cms.gov/ActuarialStudies/downloads/Last_ Year_of_Life.pdf. [Google Scholar]
- 63.Lawrence WF, Peshkin BN, Liang W, Isaacs C, Lerman C, Mandelblatt JS. Cost of genetic counseling and testing for BRCA1 and BRCA2 breast cancer susceptibility mutations. Cancer Epidemiol Biomarkers Prev. 2001;10(5):475–481. [PubMed] [Google Scholar]
- 64.Network NCC NCCN Practice Guidelines in Oncology [Accessed 5 Dec 2009];Genetics/familial high-risk assessment: breast and ovarian. V.1.2010. http://www.nccn.org/professionals/physician_gls/PDF/genetics_screening.pdf.
- 65.U.S. Department of Labor, Bureau of Labor Statistics [Accessed 5 Dec 2009];Consumer price index-medical care, all urban consumers (US city average, not seasonally adjusted) 2009 http://data.bls.gov/cgi-bin/print.pl/news.release/cpi.nr0.htm.
- 66.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14(3):259–265. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


