Abstract
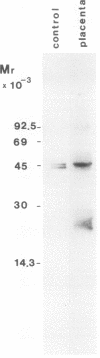
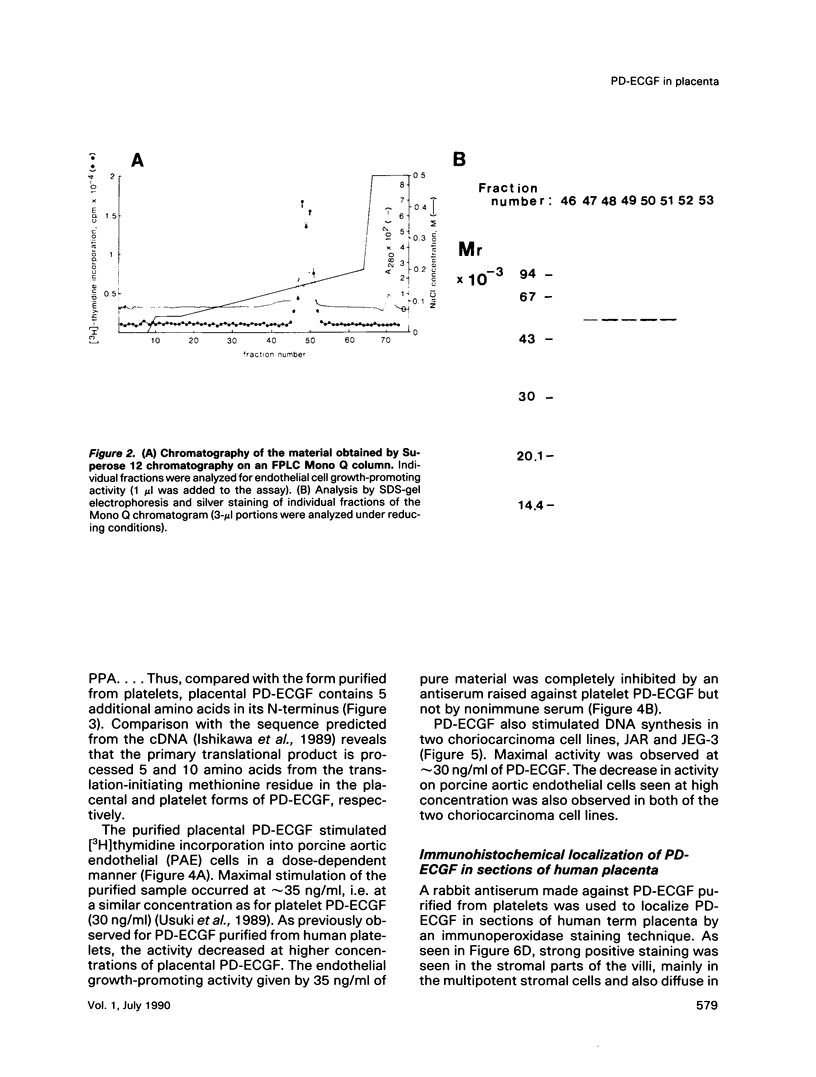
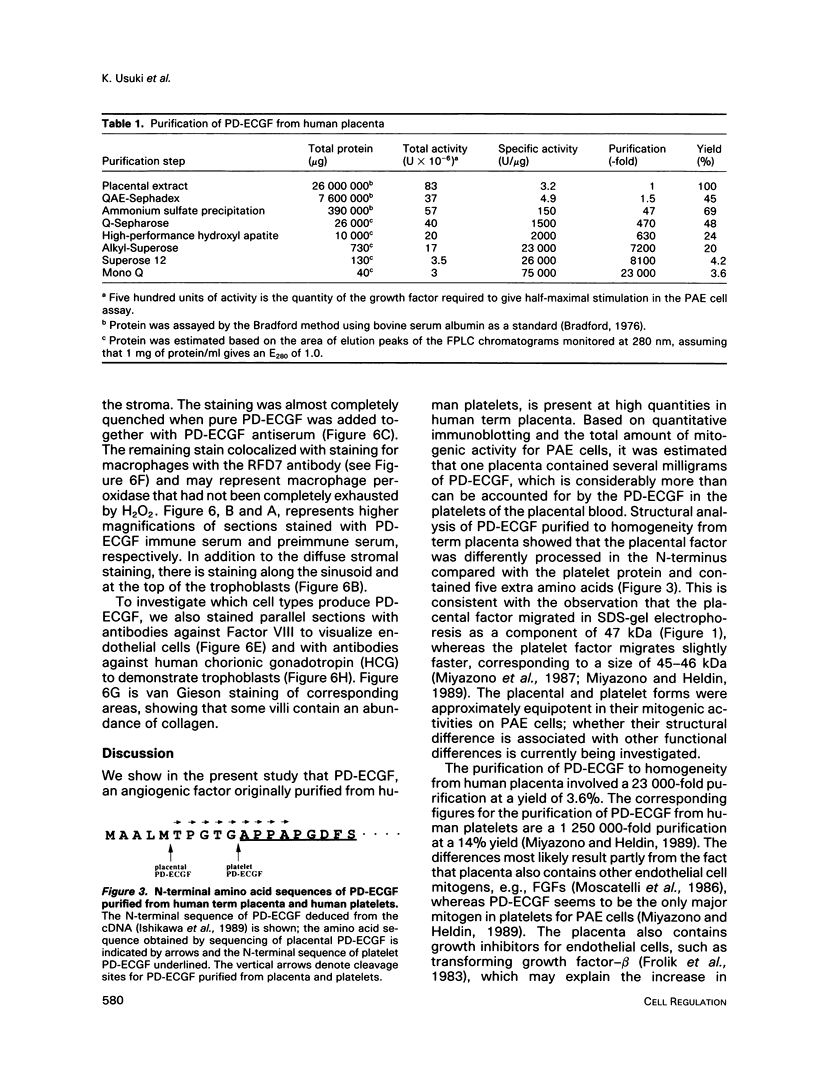
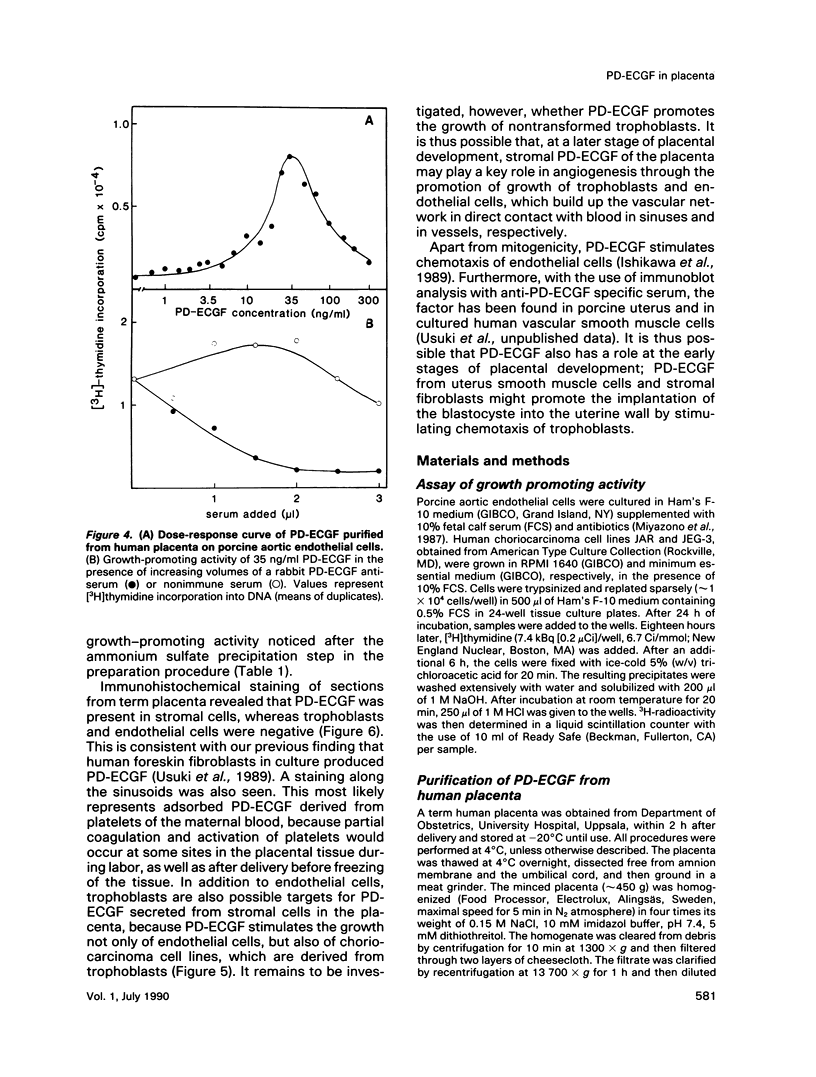
Platelet-derived endothelial cell growth factor (PD-ECGF) was purified to homogeneity from human term placenta, an organ characterized by extensive angiogenesis. N-terminal amino acid sequencing revealed that placental PD-ECGF was proteolytically processed at Thr-6, in contrast to PD-ECGF purified from human platelets, which is processed at Ala-11. The purified factor stimulated porcine aortic endothelial cells as well as two choriocarcinoma cell lines. Immunohistochemical staining revealed that PD-ECGF was present in the connective tissue cells of the placenta. The possibility that PD-ECGF is involved in the development of the placenta is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm K. D., Kelley M. F., Ilan J., Ilan J. The interleukin 2 gene is expressed in the syncytiotrophoblast of the human placenta. Proc Natl Acad Sci U S A. 1989 Jan;86(2):656–660. doi: 10.1073/pnas.86.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Flynn A., Finke J. H., Hilfiker M. L. Placental mononuclear phagocytes as a source of interleukin-1. Science. 1982 Oct 29;218(4571):475–477. doi: 10.1126/science.6981846. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Dart L. L., Meyers C. A., Smith D. M., Sporn M. B. Purification and initial characterization of a type beta transforming growth factor from human placenta. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3676–3680. doi: 10.1073/pnas.80.12.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goustin A. S., Betsholtz C., Pfeifer-Ohlsson S., Persson H., Rydnert J., Bywater M., Holmgren G., Heldin C. H., Westermark B., Ohlsson R. Coexpression of the sis and myc proto-oncogenes in developing human placenta suggests autocrine control of trophoblast growth. Cell. 1985 May;41(1):301–312. doi: 10.1016/0092-8674(85)90083-2. [DOI] [PubMed] [Google Scholar]
- Ishikawa F., Miyazono K., Hellman U., Drexler H., Wernstedt C., Hagiwara K., Usuki K., Takaku F., Risau W., Heldin C. H. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989 Apr 13;338(6216):557–562. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- Kaplow I. S. Letter: Substitute for benzidine in myeloperoxidase stains. Am J Clin Pathol. 1975 Mar;63(3):451–451. doi: 10.1093/ajcp/63.3.451. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Heldin C. H. High-yield purification of platelet-derived endothelial cell growth factor: structural characterization and establishment of a specific antiserum. Biochemistry. 1989 Feb 21;28(4):1704–1710. doi: 10.1021/bi00430a042. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Okabe T., Urabe A., Takaku F., Heldin C. H. Purification and properties of an endothelial cell growth factor from human platelets. J Biol Chem. 1987 Mar 25;262(9):4098–4103. [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Rifkin D. B. Purification of a factor from human placenta that stimulates capillary endothelial cell protease production, DNA synthesis, and migration. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2091–2095. doi: 10.1073/pnas.83.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson R. Growth factors, protooncogenes and human placental development. Cell Differ Dev. 1989 Oct;28(1):1–15. doi: 10.1016/0922-3371(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Holmgren L., Glaser A., Szpecht A., Pfeifer-Ohlsson S. Insulin-like growth factor 2 and short-range stimulatory loops in control of human placental growth. EMBO J. 1989 Jul;8(7):1993–1999. doi: 10.1002/j.1460-2075.1989.tb03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter L. W., Campbell D. A., Munro C., Janossy G. Discrimination of human macrophages and dendritic cells by means of monoclonal antibodies. Scand J Immunol. 1986 Sep;24(3):351–357. doi: 10.1111/j.1365-3083.1986.tb02104.x. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuki K., Heldin N. E., Miyazono K., Ishikawa F., Takaku F., Westermark B., Heldin C. H. Production of platelet-derived endothelial cell growth factor by normal and transformed human cells in culture. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7427–7431. doi: 10.1073/pnas.86.19.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Daimon M., Shen S. J., Engelmann G. L., Ilan J. Insulin-like growth factor-I messenger ribonucleic acid in the developing human placenta and in term placenta of diabetics. Mol Endocrinol. 1988 Mar;2(3):217–229. doi: 10.1210/mend-2-3-217. [DOI] [PubMed] [Google Scholar]